Updates
Notice
February 2026
Effective January 1, 2026, the Ohio Department of Aging has implemented important updates to State Plan Medicaid that directly impact Durable Medical Equipment (DME) providers across the state. Lift recliners—previously not covered under State Plan Medicaid—are now eligible for coverage when medically necessary. These changes create new opportunities for DME providers while shifting how requests for this equipment must be submitted and authorized.
Key Change: Lift Recliners Now Covered Under the DME Benefit
Beginning January 1, 2026, medically necessary lift recliners are included in Medicaid’s DME benefit. Providers will need to follow the State Plan Medicaid process rather than waiver-based pathways.
Previously, lift recliners could only be provided to individuals receiving home- and community-based waiver services, but this is no longer permitted unless a rare emergency exception applies.
Action Steps for DME Providers
To ensure compliance and timely payment, providers should immediately adjust internal processes:
1. Stop Submitting Waiver Program Requests
- Requests for lift recliners through waiver programs must not be submitted for any date of service after January 1, 2026.
2. Use the State Plan Prior Authorization (PA) Process
All lift recliner requests must now flow through Medicaid’s DME prior authorization channel. Submissions must include:
- Documentation of medical necessity
- One of the following HCPCS codes for the lift mechanism:
- E0627
- E0629
- Plus:
- A9900 U1 (now added to the DME tab)
3. Ensure Billing Accuracy with Updated Payment Rates
The memo outlines the following Medicaid payment amounts:
Recliner Type | HCPCS Codes | Total Payment |
Electric lift recliner | E0627 + A9900 U1 | $1,263.48 |
Non-electric lift recliner | E0629 + A9900 U1 | $1,260.37 |
Note: Higher amounts may be considered when a heavy‑duty lift recliner is medically necessary.
Additionally:
- HCPCS codes E0627 and E0629 have been moved from the atypical tab to the DME tab.
- A9900 U1 is now officially listed under the DME tab as well.
Waiver Program Impact
Lift recliners should not be authorized through waiver programs except in very limited circumstances.
An exception may be granted only in documented emergencies where a delay in equipment would place a member’s health or safety at significant risk.
Background
Prior to 2026, State Plan Medicaid did not cover lift recliners at all. This expansion allows broader access for Medicaid members while creating a standardized, statewide path for DME providers to supply this equipment.
Need Help or Have Questions?
Questions regarding these updates should be directed to:
Further regulatory detail is available in the Ohio Administrative Code:
🔗 OAC 5160‑10‑01 (DME rule)
January 2026
Ohio Department of Medicaid to Update OhioRISE Eligibility & Enrollment Rule
The Ohio Department of Medicaid (ODM) intends to amend the OhioRISE: eligibility and enrollment rule, 5160-59-02, so that enrollment in OhioRISE will become effective the first day of the calendar month of eligibility. This change is being made to align with managed care program enrollment and to reduce manual retro-enrollments due to an inpatient behavioral health admission. The rule change is intended to be effective July 1, 2026.
Clarification of financial responsibility for behavioral health services provided to children and youth is found in the OhioRISE Mixed Services Protocol (Protocol) link. The Protocol will be updated as needed to support the rule change.
The Ohio Department of Health (ODH) implemented licensure requirements for all Home Health Agencies (HHAs) beginning Sept. 30, 2022. At that time, agencies were issued triennial licenses set to expire in the fall of 2025.
Despite extensive outreach - including communication with associations, coordination with other state entities, and posting expiration dates on the ODH website - a significant number of licensed HHAs did not submit timely renewal applications. This lapse places reimbursement eligibility at risk for affected agencies.
To address this issue and support compliance with Ohio's licensure requirements, ODH is granting a one-time grace period for renewals. Any HHA whose license was due for renewal between July 31, 2025, and Dec. 31, 2025, will have until Feb. 1, 2026, to file for renewal. Licenses renewed during this grace period will remain active as of their original expiration date.
Beginning Feb. 1, 2026, ODH will provide advance notice of upcoming expiration dates and renewal requirements to each HHA. However, any HHA that fails to renew timely after this date will be required to restart the licensing process by applying for a new initial license.
If you have already submitted your renewal application, please disregard this message.
For questions or assistance with the renewal process, please contact ODH at (614) 466-7713 or visit Home Health Agencies on the ODH website.
December 2025
As we come to the close of 2025, Buckeye thanks you for your partnership in providing quality care and service to our members to improve health outcomes. Our strong partnership is crucial managing costs, and ensuring high-quality, efficient healthcare delivery. Through collaboration, we have shared knowledge and resources to address complex patient needs and align quality goals with payment structures. By working together, we have enhanced communication and supported each other’s efforts to put our members’ well-being at the heart of everything we do. We are honored to work alongside you to bring healing and well-being to those we serve.
We hope you enjoy a wonderful holiday season and look forward to continuing our partnership in the New Year.
The Ohio Department of Medicaid (ODM) began rolling out EVV claims adjudication earlier this year, March 1, 2025, through a phased implementation approach. The next phase for EVV claims adjudication begins January 1, 2026, for providers billing for PASSPORT Waiver services.
This means, claims submitted for PASSPORT Waiver EVV services provided on or after January 1, 2026, must have a matching EVV visit record for claims to pay. Claims that do not have a matching EVV visit will be denied.
PASSPORT Waiver EVV services include:
| Service Description | Service Code |
|---|---|
| Personal Care Services | T1019 |
| Waiver Nursing | T1002, T1003 |
| Home Care Attendant Services | S5125 |
| Choices Home Care Attendant | T2025 |
| Enhanced Community Living | T2025 UA U1 |
*If you only provide participant-directed Choices Home Care Attendant Service and submit your hours through the Fiscal Management Service (PPL), please contact the FMS directly if you have any questions.
Find out how you’re doing with claims adjudication by visiting ODM’s EVV Provider Dashboard which is now updated with data on claim lines since July 2024 with paid dates after September 2024. The dashboard allows you to see the number of claim lines and dollar amounts associated with specific EVV errors. For assistance navigating the dashboard, review the provider lookup quick reference guide.
ODM is working with HHAeXchange (formerly Sandata), an EVV vendor, to make sure you are ready for this change. We strongly encourage you to reach out for assistance and support now:
- Daily office hours, offering 1:1 support.
- A complete list of HHA-Sandata Phase 6 learning sessions.
- Contact HHAeXchange-Sandata.
- EVV Claims Processing Flow Chart for Department of Aging (AGE) providers.
Providers can find the latest EVV tools and resources on Ohio Medicaid’s Electronic Visit Verification webpage.
Medicare has extended its current telehealth flexibilities through January 30, 2026. At this time, it is unknown whether these flexibilities will be extended further. Regardless of future Medicare decisions, Wellcare By Allwell Medicare plans will continue to offer expanded telehealth access through 2026 plan years. Our benefits maintain many of the same flexibilities, ensuring no change in telehealth coverage for Wellcare By Allwell’s Medicare members.
Current Medicare telehealth flexibilities include:
- Relaxed geographic restrictions
- No originating-site requirements
- Expanded list of eligible telehealth providers
Wellcare By Allwell Medicare plans will continue these allowances even if Medicare ends its flexibilities:
- Members may receive telehealth services from any location—no rural or originating-site limitations.
- No restrictions on which types of providers may deliver telehealth.
- Teladoc services remain available 24/7 at $0 cost share.
- Telehealth from in-network providers applies the same cost share as an in-office visit (e.g., PCP or specialist rates). Members should refer to their EOC for full cost-share details.
- No technology requirement—audio-only telehealth is permitted for certain non-behavioral, non-mental health services.
If you have any questions, please contact If you have any questions, please contact Provider Services.
In January 1, 2026, Wellcare offers multiple types of dual special needs plans (D-SNPs) to Ohioans. We're providing you with some key differences between these D-SNP designs and will help you perform common transactions depending on which plan the member is enrolled. See our document.
Managing Duals Digitally – Frequently Asked Questions
In 2026, Wellcare By Buckeye Health Plan offers an integrated dual special needs plan (D-SNP) for members eligible for both Medicare and Medicaid. Managing care for these Duals members in our digital tools will remain familiar with similar workflows you rely on today for other lines of business, plus some enhancements for more integrated views across Medicaid and Medicare benefits. See the Duals FAQs.
Per Ohio Department of Medicaid (ODM) guidelines, diagnoses reported solely on radiology, pathology or laboratory claims are not eligible for Risk Adjustment Factor (RAF) calculations. When these services are billed without an accompanying professional claim, valid CDPS conditions may not be captured, resulting in missing diagnosis gaps. To support accurate RAF reporting and diagnosis gap closure, providers must submit a separate professional claim in addition to the facility claim when laboratory, radiology, or pathology services are rendered.
On January 1, 2026, the Centers for Medicare & Medicaid Services (CMS) will implement new prior authorization (PA) response time requirements for all providers.
For Medicaid & Medicare:
- Standard prior authorization requests will be completed within 7 calendar days, with a possible extension up to 14 calendar days under certain circumstances.
- Expedited/Urgent prior authorization requests will be completed within the lesser of 48 hours -OR- 2 calendar days.
With shorter response times for supporting clinical information requests, all necessary clinical information should be submitted at the time of the authorization request.
Additional Information
- Complete clinicals include Diagnosis, History and Current Condition, Treatment Plan and Interventions, and Relevant Diagnostic Tests.
- Response times can be lessened if all information is submitted with the authorization request.
- Missing clinical information may lead to a denial due to inadequate supporting records.
- Submitting prior authorization requests via the secure Availity portal allows for faster review.
As part of the 2026 Duals Operating Model, providers in Amisys will need to select and add the new Duals line of business in Payspan to receive payments through their preferred payment method.
New providers must complete full registration.
Payspan Contact Information:
Payspan Provider Relations (877) 331-7154, Option 1.
Failure to add the Duals line of business will result in providers receiving payment via paper check. www.payspanhealth.com
CPT codes G2211 & G2212 will no longer be covered by Ambetter beginning on Apr. 1, 2026. For Details.
As part of our continued commitment to supporting providers and ensuring compliance with State and CMS requirements, we are implementing important system changes aligned with the Dual Special Needs Plan (D-SNP) offering, effective January 1, 2026.
We are working to implement Pre-Payment Edits associated with the D-SNP product. These updates will enhance claim accuracy, strengthen policy compliance, and streamline payment processing.
Temporary Process: Post Payment Edits
While implementation work continues, Pre-Payment Edits are expected to go live in early 2026. Until that time, select claims may be subject to Post-Payment Edits rather than pre-payment validations, unless otherwise stated in your provider agreement.
This approach allows payments to continue without disruption while maintaining oversight through post-payment review. When the edits are activated, any claims paid incorrectly according to policy may be subject to recoupment under standard procedures.
Provider Responsibilities and Next Steps
We appreciate your partnership during this transition and ask that you please review the following reminders:
- Providers remain responsible for submitting complete, accurate, and timely billing information to ensure correct payment. Continue to submit claims per the existing guidelines outlined in your provider manual.
- Payment Integrity teams may issue additional reminders or updates as we approach go-live.
- If you have any questions or concerns, please reach out to the Provider Relations team.
We value your continued collaboration and partnership as we implement these improvements to ensure accuracy, efficiency, and compliance with claims processing.
November 2025
On January 1, 2026, the Centers for Medicare & Medicaid Services (CMS) will implement new prior authorization (PA) response time requirements for all providers.
For Medicaid & Medicare:
- Standard prior authorization requests will be completed within 7 calendar days, with a possible extension up to 14 calendar days under certain circumstances.
- Expedited/Urgent prior authorization requests will be completed within the lesser of 48 hours -OR- 2 calendar days.
With shorter response times for supporting clinical information requests, all necessary clinical information should be submitted at the time of the authorization request.
Additional Information
- Complete clinicals include Diagnosis, History and Current Condition, Treatment Plan and Interventions, and Relevant Diagnostic Tests.
- Response times can be lessened if all information is submitted with the authorization request.
- Missing clinical information may lead to a denial due to inadequate supporting records.
- Submitting prior authorization requests via the secure Availity portal allows for faster review.
REMINDER:
No Balance Billing. Pursuant to OAC rule 5160-26-05, Provider shall not bill Covered Persons any amount greater than would be owed if the entity provided the Covered Services directly (i.e., no balance billing). (SC App. B., §4.h.) We are aware that several factors can impact a quick, full reimbursement from multiple sources. Medicaid providers are prohibited from billing members in any situation per ODM . This guidance is found in our Provider Manual on page 74, as well as the Provider Contract found on the NextGen MyCare Contracted Providers page.
We previously shared information about participation in our new Ambetter offering: Ambetter Health Solutions. This individual product, similar to Ambetter Premier, is designed for those using an Individual Coverage Health Reimbursement Arrangement (ICHRA) to purchase coverage. You will continue to use the same portals and processes for Solutions members as you do for Ambetter members—no additional steps are required.
For more details, visit this link:
Ambetter's ICHRA Homepage This hub provides an overview of ICHRA coverage and resources for employers, brokers, and employees.
As part of the 2026 Duals Operating Model, providers in Amisys will need to select and add the new Duals line of business in Payspan to receive payments through their preferred payment method.
New providers must complete full registration.
Payspan Contact Information:
Payspan Provider Relations (877) 331-7154, Option 1.
Failure to add the Duals line of business will result in providers receiving payment via paper check. www.payspanhealth.com
As indicated in ORC 3129.06, coverage of the following medications under medical benefit for the Medicaid line of business is prohibited for individuals under the age of 18 for use in gender dysphoria or gender transition.
- Goserelin Acetate (Zoladex)
- Histrelin Acetate (Vantas, Supprelin LA)
- Leuprolide Acetate (Eligard, Fensolvi, Lupron Depot, Lupron Depot-Ped), Leuprolide Mesylate (Camcevi)
- Nafarelin Acetate (Synarel)
- Triptorelin Pamoate (Trelstar, Triptodur)
- Testosterone (Testopel, Jatenzo, Kyzatrex)
Per ORC 3129.06, exceptions to coverage, which do not prohibit a physician from treating, including by performing surgery on or prescribing drugs or hormones for, a minor individual who meets the following criteria:
- Individual was born with a medically verifiable disorder of sex development, including an individual with external biological sex characteristics that are irresolvably ambiguous, such an as individual born with forty-six XX chromosomes with virilization, forty-six XY chromosomes with undervirilization, or having both ovarian and testicular tissue;
- Individual received a diagnosis of a disorder of sexual development, in which a physician has determined through genetic or biochemical testing that the individual does not have normal sex chromosome structure, sex steroid hormone production, or sex steroid hormone action for a biological male or biological female;
- Individual needs for any infection, injury, disease, or disorder that has been caused or exacerbated by the performance of gender transition services, whether or not the services were performed in accordance with state or federal law;
- Mental health services provided for a gender-related condition;
- Any services that are not gender transition services.
These changes affect the following site of care settings: provider-administered, outpatient hospital or ambulatory care center. To obtain forms for authorizations, please visit Buckeye’s Forms Website Page or contact Provider Services at 866-296-8731.
Thank you for your partnership and for the quality care you provide to Buckeye members.
Buckeye makes sure you our members have access to their Medicaid plan benefits and information at their fingertips. With our free mobile app, they can:
- Instantly access member ID Card
- View plan benefits
- Search for a doctor, specialist or pharmacist in their area
- And more!
Members can learn more about the app and find links to download it to their Apple or Android smartphones at www.buckeyehealthplan.com/app
October 2025
The following are high-impact Part-D formulary changes that will affect the Medicare Advantage and MyCare lines of business in 2026:
Therapeutic Class | Drug Removed | Formulary Alternative(s) & Restriction(s) |
Insulin, Long-Acting | INSULIN DEGLUDEC U-100, U-200 | INSULIN GLARGINE-yfgn pen & vial, INSULIN GLARGINE U-300 pen |
Topical NSAID | diclofenac 2% topical solution pump | diclofenac 1.5% topical solution (QL) |
Movement Disorders | AUSTEDO, AUSTEDO XR | INGREZZA (PA, QL), tetrabenazine (PA, QL) |
Constipation | TRULANCE | LINZESS (QL), lubiprostone (QL) |
GLP-1/Insulin Combination | XULTOPHY | SOLIQUA (QL) |
Asthma Biologic | FASENRA | DUPIXENT (PA,QL), XOLAIR (PA,QL) |
Glucagon-like Peptide 1 Agonist | BYDUREON BCISE | MOUNJARO (PA, QL), OZEMPIC (PA, QL), RYBELSUS (PA, QL), TRULICITY (PA, QL) |
Please note this is not a complete list of formulary changes. Please consult list of drugs change notices for further information.
The following are the preferred biosimilars for the Medicare Advantage and MyCare lines of business in 2026:
DRUG CLASS | FORMULARY CHANGE | PREFERRED BIOSIMILAR |
Inflammatory Disorders | Remove HUMIRA (adalimumab)** | CYLTEZO (adalimumab-adbm)** [PA, QL], YUFLYMA (adalimumab-aaty)** [PA, QL] |
Inflammatory Disorders | Remove ACTEMRA SC (tocilizumab) | TYENNE SC (tocilizumab-aazg) [PA, QL] |
Inflammatory Disorders | PA restriction on STELARA (ustekinumab)** to try biosimilar alternative | STEQEYMA (ustekinumab-stba)** [PA, QL] |
Inflammatory Disorders | PA restriction on ENBREL (etanercept) to try 1 adalimumab alternative | CYLTEZO (adalimumab-adbm) [PA, QL], YUFLYMA (adalimumab-aaty) [PA, QL] |
Please note this is not a complete list of formulary changes. Please consult list of drugs change notices for further information.
As we approach the final quarter of the year, we’re entering a critical phase of the Continuity of Care (CoC) program. Our shared goal remains clear: to ensure patients receive timely, high-quality care while closing the most impactful clinical gaps by December 31st. To support you in this effort, we’ve refined the appointment agenda to focus on the most urgent and actionable opportunities with a particular emphasis on risk adjustment conditions. This streamlined approach is designed to:
- Prioritize the highest-value clinical care gaps for closure;
- Maximize your time during each patient encounter to focus on the more critical clinical and care items; and
- And ensure documentation efforts are aligned with confirmed and clinically relevant conditions.
By concentrating on these key areas, we can collectively improve care quality, enhance patient outcomes, and ensure accurate risk adjustment data capture.
We appreciate your continued partnership and dedication to delivering high-quality, patient-centered care. If you have any questions or need support, please reach out to your Provider Engagement Administrator.
Thank you for your dedication and collaboration.
In our June Provider Bulletin we announced a partnership with tango|WellSky to deliver comprehensive Skilled Home Health and Post-Acute Facility management services for our Medicare Advantage members. As we move closer to the targeted effective date of October 1, 2025, we wanted to let you know that you may hear directly from tango as they build the Home Health network to support the Medicare Advantage product. The agreement signifies the appointment of tango – WellSky as the delegated manager for skilled home health and post-acute care benefits related to skilled nursing facilities, inpatient rehabilitation facilities, and long-term acute care hospitals specifically for the Medicare Advantage members in our Wellcare by Allwell program This partnership is specific to Wellcare by Allwell’s Medicare Advantage members in the following plans:
- HMO
- HMO - Dual Special Needs Plan
In this partnership, tango is appointed as the delegated manager for skilled home health benefit for our Medicare Advantage members. The expanded partnership encompasses several key components that aim to enhance the quality and delivery of skilled home health care for Medicare Advantage members:
- Network Development and Management:
- Developing, maintaining, and credentialing a robust network of skilled home health care providers.
- Referral Coordination and Authorization Management:
- Efficient management of skilled home health referrals, referral coordination, and authorizations.
- Claims Payment:
- Timely and accurate claims payment for the services provided under this delegation.
- Buckeye will continue to directly contract and pay claims for Post-Acute Facilities
You can learn more about contracting with tango by reaching out to tango’s dedicated team using one of the following options:
- By phone: 1-888-224-1409
- Website: https://providerresourcecenter.com/wellcare/External Link
- Email: contractmanagement@tangocare.com
If you have further questions, feel free to reach out to your Buckeye Provider Engagement Account Manager.
September 2025
Centene Health Plans recently went live with Availity’s Auth Inquiry Expansion feature(s) for all lines of business.
If you have any questions, please contact Provider Services at 866-296-8731 or your Provider Engagement Administrator.
The Ohio Department of Medicaid (ODM) sent out a Medicaid Advisory Letter in July 2025 regarding the U.S. Department of Health and Human Services sterilization consent form (HHS-687).
The HHS-687 form had an expiration date of 07/31/2025 and ODM advised to continue to accept the current form until further notification.
The HHS-687 form has now been updated with an expiration date of 07/31/2028.
Links to HHS-687 form:
https://opa.hhs.gov/sites/default/files/2025-07/consent-for-sterilization-english-2025.pdf
https://opa.hhs.gov/sites/default/files/2025-07/consent-for-sterilization-spanish-2025.pdf
Beginning January 1, 2026, we will launch an aligned Dual Special Needs Plan (D-SNP) in Ohio. As directed by CMS, our current Medicare-Medicaid Plan (MMP), MyCare, will sunset on December 31, 2025, and members will be automatically transitioned into the new aligned D-SNP, Wellcare by Buckeye Health Plan.
Like the MMP, this new plan is designed for individuals who qualify for both Medicare and Medicaid, allowing their benefits to be coordinated and managed by a single healthcare organization.
To help you prepare for this transition, we invite you to explore the tools and resources available on our NextGen MyCare Contracted Provider Page. From there, you can access:
- A link to register for the secure provider portal
- The Provider Manual and Quick Reference Guide(s)
If you have additional questions about this change, your contract status, or to join our network, please contact Provider Services at 1-833-998-4892.
The Ohio Department of Medicaid Next Generation MyCare program is an improved healthcare program for Ohioans who have both Medicaid and Medicare that helps members get the care they need all in one plan. The Plan gives members all benefits available through the traditional Medicare and Medicaid programs, including long-term care services both in the community, assisted living, and in a nursing facility as well as behavioral health services. We are pleased to share with you the launch of the Buckeye Health Plan website pages on October 1, 2025, that support this new product starting January 1, 2026.
The Ohio Department of Medicaid (ODM) Substance Use Disorder Notification of Admission Form is a tool for SUD providers to notify the appropriate Managed Care Entity (MCE) within 48 hours of a Medicaid member’s admission.
Once received, the MCE will complete Section III of the form and return it to the provider within 24 hours.
This process ensures timely coordination and documentation of care. Please ensure your teams are aware of this and are submitting forms within the designated timeframe.
For Buckeye Medicaid members, please submit this form via email to BuckeyeSUDNotification@centene.com
You can find the Substance Use Disorder Residential Treatment Notification of Admission Form with the contact information for submission at medicaid.ohio.gov/static/Resources/Publications/Forms/ODM10294Fillx.pdf.
Free Accredited Training for Providers
Pediatric Suicide Prevention for Primary Care Providers
Sept. 30, 1:00 – 2:00 p.m. ET
Suicide is preventable, and no one is beyond help. Healthcare providers play a vital role in identifying individuals at risk early on, and primary care serves as a crucial touchpoint. To help combat this unnecessary loss of life, Buckeye Health Plan is offering a free accredited continuing education course titled Pediatric Suicide Prevention for Primary Care Providers. This training is designed to equip you with the knowledge and tools needed to recognize and support individuals at risk, enabling timely and effective intervention.
Join the Association of Clinicians for the Underserved (ACU) in collaboration with Buckeye Health Plan and Centene Institute for this LIVE training session. Presenters will highlight the important role of providers in suicide prevention and discuss common warning signs and risk factors, detail effective pediatric suicide prevention practices, and review clinical pathways and evidence-based interventions.
Why Take This Course?
- Free and Accredited. This course is available at no cost and offers 1.00 continuing education credits for physicians, nurses, social workers, and psychologists.
- On Demand Education. Delivered virtually, this comprehensive course will strengthen your ability to identify at-risk individuals early on. Highlights include insight into suicide risk, assessment strategies, youth risk factors, and review clinical pathways and evidence-based interventions.
- Immeasurable Impact. Help us save lives in the communities we serve.
Buckeye recognizes that increased access to suicide prevention saves lives. Your partnership and participation can make a significant impact to help individuals live their lives to the fullest.
How to Enroll
Registration at this Zoom link or http://bit.ly/47YUIyi is required to attend. This course is accredited by Centene Institute for Advanced Health Education and offered in partnership with Buckeye. CME credits are available for physicians, nurses, social workers, and psychologists.
Questions?
Contact your Provider Engagement Administrator or Provider Services at 866.296.8731
August 2025
As we move into the end of 2025, we want to be sure you are aware that the WellCare by Allwell logo being used for 2025 will be just WellCare in 2026. You may find you receive materials from Centene, WellCare, or WellCare by Allwell teams in 2025 with the 2026 WellCare logo. If you have any questions on this transition, please reach out to Provider Services.


As we move into the end of 2025, we want to be sure you are aware that the Ambetter from Buckeye Health Plan logo being used for 2025 will be just Ambetter or Ambetter Health in 2026. You may find you receive materials from Centene, Ambnetter, or Ambetter from Buckeye Health Plan teams in 2025 with the 2026 Ambetter or Ambetter Health logo. If you have any questions on this transition, please reach out to Provider Services.


ODM Care Management Newsletter - 3rd Q 2025 Back to School Edition
To ensure ODM’s continued compliance with American Rescue Plan Act Requirements (Section 9817) Buckeye Health Plan must prior authorize Assertive Community Treatment (ACT), Intensive Home-based Treatment (IBHT), and Substance Use Disorder (SUD) Residential Treatment (beginning with the third calendar stay) no later than 48 hours after receipt of the request. To aid Buckeye Health Plan in identifying these requests we encourage providers to mark them as Urgent/Expedited when they are submitted. OUTPATIENT MEDICAID PRIOR AUTHORIZATION FAX FORM OH-PAF-0672 OP You'll find this in our Forms section.
July 2025
As a participating Primary Care Provider (PCP) in Buckeye Health Plan’s Medicaid Managed Care Network you have responsibilities that include oversight of home and community-based services (HCBS). This oversight includes personal care aide support, as outlined by the Ohio Department of Medicaid (ODM).
Under Ohio Medicaid’s managed care framework and per ODM requirements, PCPs play a critical role in determining and authorizing the medical necessity of home care services. These services must be part of a comprehensive, person-centered care plan and aligned with each member’s functional needs and clinical condition. We want to remind you of your responsibilities in evaluating and certifying medical necessity for HCBS which includes:
- Home health aide or personal care support.
- Documenting clinical justification for all HCBS referrals in the member’s medical record and care plan.
- Monitoring changes in condition that may warrant continuation, reduction, or termination of home care services.
- Avoiding overutilization by ensuring members meet clinical and functional thresholds for this level of support.
Our partnership places strong emphasis on accountability and care coordination to ensure services are effective, efficient and appropriate for each member’s needs. Buckeye Health Plan’s utilization review processes may include audits of medical necessity determinations made by PCPs and providers may be asked to submit documentation supporting referrals for home care services.
We encourage you to work closely with Buckeye’s Utilization Management Team to ensure that your patients’ home care services are aligned with Buckeye’s policy expectations. If you have questions about HCBS criteria, care plan development or documentation requirements, please reach out to us at OH_UM_Questions@CENTENE.COM.
Thank you for your continued commitment to our members and your role in maintaining high standards of care.
The Ohio Department of Medicaid (ODM) is providing guidance regarding U.S. Department of Health and Human Services sterilization consent form (HHS-687). The HHS-687 form is used as informed consent for sterilization as documented in Ohio Administrative Code Rule 5160-21-02.2. ODM is aware this form has an expiration date of July 31, 2025, but please continue to use the current HHS-687 until further notice.
Secondary ventilator requirements – Buckeye Health Plan requires a properly completed and approved Certificate of Medical Necessity (CMN) form to be submitted with the secondary vent claim in order to cover and pay for a secondary ventilator unit. Failure to submit a complete and accurate form with the claim will result in denial of payment for this item. For more information on this requirement, please refer to section B(4) in the following OAC Rule: https://codes.ohio.gov/ohio-administrative-code/rule-5160-10-22
In our June Provider Bulletin we announced a partnership with tango|WellSky to deliver comprehensive Skilled Home Health and Post-Acute Facility management services for our Medicare Advantage members. As we move closer to the targeted effective date of October 1, 2025, we wanted to let you know that you may hear directly from tango as they build the Home Health network to support the Medicare Advantage product.
In this partnership, tango is appointed as the delegated manager for skilled home health benefit for our Medicare Advantage members. The expanded partnership encompasses several key components that aim to enhance the quality and delivery of skilled home health care for Medicare Advantage members:
- Network Development and Management:
- Developing, maintaining, and credentialing a robust network of skilled home health care providers.
- Referral Coordination and Authorization Management:
- Efficient management of skilled home health referrals, referral coordination, and authorizations.
- Claims Payment:
- Timely and accurate claims payment for the services provided under this delegation.
- Buckeye will continue to directly contract and pay claims for Post-Acute Facilities
You can learn more about contracting with tango by reaching out to tango’s dedicated team using one of the following options:
- By phone: 1-888-224-1409
- Website: https://providerresourcecenter.com/wellcare/
- Email: contractmanagement@tangocare.com
If you have further questions, feel free to reach out to your Buckeye Provider Engagement Account Manager.
June 2025
In preparation for the January 1, 2026, change in the Ohio Department of Medicaid turnaround times, Buckeye is turning faxes off to providers for initial Inpatient scheduled and emergent admissions as well as all Prior Authorization requests beginning July 1, 2025. We are asking providers begin using only the Buckeye Portal or Availity to submit the above mentioned initial requests and their supporting clinical.
Buckeye Health Plan will continue to accept continued stay inpatient clinical and discharges via fax at 866-529-0290.
If this requirement cannot be met due to financial hardship, including limited internet connection or no availability, please contact Buckeye so that alternate arrangements can be made. Submit your request here.
In a continuous effort to make it easier to do business with us, Wellcare by Allwell is introducing Availity Editing Services (AES). Starting August 16, 2025, and running through the end of 2025, Centene is partnering with Availity to return rejection messages on its behalf via AES messages.
These messages will show in your existing workflows. AES will give you an option, but not a requirement, to edit a claim.
AES can identify a claim error upfront and return a message to you for correction before sending the claim on to the plan to be adjudicated. You should review edit messages for potential corrections to the suggested claim line(s). If you make updates to the claim, this may help the claim process correctly the first time, preventing errors, improving payment accuracy, and claims adjudication turnaround time. If, after reviewing the message, you find it does not apply, please resubmit the claim as-is and this will allow a bypass of the edit in cases where it may not be applicable.
This is not intended as a new method to deny a claim, nor does it bypass or replace downstream edits. If you choose to bypass an edit, it is possible that other downstream edits will still function as normal in our claims systems. Remember to “submit” your claim regardless of your choice to edit or bypass. This action is required in order for the claim to be processed in our systems.
If you have a Practice Management System (PMS), you can locate your edits report within your claims workbasket or que reporting. If you submit claims via the Availity portal, any of these rejections will show on your normal reports.
If you submit claims via Availity, learn how to gather your reporting by joining one of Availity’s free webinars to learn additional tips for streamlining your workflow:
Send and Receive EDI Files – Training Demo
This demo shows users where/how they can access reports in Availity Essentials. On these reports are where they would see edits. Please note: this demo does not say/call it AES however, this is the demo that would show the user how to locate the reports.
EDI Reporting Preferences – Training Demo
This demo shows users how to setup their EDI Reporting Preferences which needs to be done first by the user’s organization’s Availity Administrator to access the reports in the Send and Receive EDI Files application.
If you need assistance with registering for Availity Essentials, please call Availity Client Services at 1-800-AVAILITY (282-4548). Assistance is available Monday through Friday, 8 a.m. – 8 p.m. ET. For general questions, please reach out to your Provider Engagement Administrator.
As part of ongoing system maintenance, Buckeye Health Plan's authorization system will be upgraded, requiring a scheduled system outage Friday, October 10 at 10 p.m. CT through Monday, October 13 at 7:00 a.m. CT.
During this scheduled outage, we will be unable to receive authorization requests except as noted below. Please resume submission of your authorization requests on Monday, October 13, 2025.
How to Plan for the Outage:
- Plan accordingly and submit any urgent or time-sensitive authorization requests prior to the scheduled downtime.
- For urgent authorization requests during the outage, call 833-837-0188. Of note, this number will only be active during this downtime.
- Beginning Monday, October 13 at 7 a.m. CT, providers may submit authorization requests as usual.
If you have questions, please contact your Provider Engagement Administrator
We are pleased to announce that effective October 1, 2025, Centene (Buckeye’s Parent Company), tango and WellSky are expanding their partnership to Ohio to deliver comprehensive Skilled Home Health and Post-Acute Facility management services for our Medicare Advantage members.
The tango – WellSky solution brings a first of its kind partnership to the market to use the leading technology and services capabilities of WellSky and the skilled home health enablement expertise of tango to deliver a fully integrated proactive post-acute care program that streamlines and coordinates a cohesive provider and member experience across the post-acute continuum.
The agreement signifies the appointment of tango – WellSky as the delegated manager for skilled home health and post-acute care benefits related to skilled nursing facilities, inpatient rehabilitation facilities, and long-term acute care hospitals specifically for the Medicare Advantage members in our Wellcare by Allwell program.
We will continue to share more details with you in our next few Provider Bulletins. Stay tuned!
May 2025
As a reminder, Ohio Department of Medicaid updated Durable Medical Equipment and Supplies Effective January 1, 2024
The following codes changed from Rent to Purchase to Purchase Only.
B9002 | E0373 | E0565 | E2508 |
B9004 | E0445 | E2500 | E2510 |
B9006 | E0470 | E2502 | E2511 |
E0371 | E0481 | E2504 | E2512 |
E0372 | E0483 | E2506 |
|
Buckeye Health Plan allowed for the billing of rentals for these items that started prior to 2024 for up to ten rental months into 2024. However, these items will be denied as “purchase only” if the first rental date is in 2024 or any rental is submitted after October, 2024 dates of service. These claims will be denied with CARC 453 and RARC N823.
Optum’s Provider Portal - Enhancing Provider Experience
We are excited to announce the launch of Optum’s new Provider Portal, designed to streamline payment integrity processes and improve provider interactions.
Key Benefits of the New Portal:
- Simplified Medical Record Management – Providers can view and upload all medical record requests in one place.
- Real-Time Status Updates – Track the status of medical record submissions with ease.
- Enhanced Communication – Access Optum’s review outcomes and rationale for better transparency.
- Go Paperless! – Providers can opt for digital communications for greater efficiency.
What’s Changing?
Providers will be directed to a new URL, found on Optum’s medical record request letters, to upload their documentation. Providers can self-register to obtain full access for enhanced features or continue as non-registered users with the same current functionality.
The portal is now live and ready for providers to use!
For any questions or support, please reach out to Optum at: pi_portal_support@optum.com
April 2025
No Faxing of Service-Based Prior Authorizations Starting July 1, 2025
Under the direction of CMS, Ohio Department of Medicaid (ODM) is reducing the turnaround time requirements for prior authorizations effective January 1, 2026. In preparation, we are asking our providers to begin using only the Buckeye Portal or Availity to submit initial Inpatient scheduled and emergent admissions as well as all Prior Authorization requests beginning July 1, 2025.
These ODM guidelines include direction that all service-based prior authorizations will be submitted through a secure electronic transmission. For these purposes, a facsimile is not considered a secure electronic transmission.
When a prior authorization is submitted electronically, Buckeye will respond to requests within forty-eight hours for urgent care services or ten calendar days for any prior authorization request that is not for an urgent care service. This does not apply to emergency services.
If this requirement cannot be met due to financial hardship, including limited internet connection or no availability, please contact Buckeye so that alternate arrangements can be made. Submit your request here.
March 2025
In partnership with Edifecs, Buckeye Health Plan is implementing the Condition Continuity Inquiry (CCI) program to support the Continuity of Care (CoC) Program and Partnership for Quality (P4P) for Medicaid. This tool identifies claims that are missing historical diagnosis codes or quality care gaps and returns them to the primary care physician (PCP) through a pre-claim submission clearing house soft edit, allowing the PCP to review. You’ll find additional information in the Payer Space in Availity.
Our goal is to strengthen healthcare partnerships by using technology to simplify processes. By clearly displaying our members' health status, providers can deliver high-level care focused on prevention, condition-specific, and chronic condition management. Let's work together to make a difference!
In our February 2025 Buckeye Provider Bulletin, we shared “Effective April 1, 2025, Beyfortus (nirsevimab-alip), an FDA approved drug for RSV, will require prior authorization for utilization in the Medicaid population when billed as a medical claim. See the relevant CPT codes.” We want to clarify that Beyfortus (nirsevimab-alip) will require prior authorization for utilization in the Medicaid population when billed as a medical claim for non-Vaccines for Children (VFC) providers . There will not be any changes to coverage prior to April 1st. The administration component for the vaccine product will still be covered for VFC providers.
In preparation for the January 1, 2026, change in the Ohio Department of Medicaid turnaround times, we are asking that providers begin using only the Buckeye Portal or Availity to submit service-based prior authorizations as of July 1, 2025. We will have more details and direction in next month’s Buckeye Provider Bulletin.
Medical Policy UPDL Alignment for Certain Biopharmacy Drugs
You can find UPDL criteria at Gainwell or Ohio Medicaid Pharmacy Program
| Abatacept (Orencia) | OH.PHAR.241 |
| Benralizumab (Fasenra) | OH.PHAR.373 |
| Certolizumab pegol (Cimzia) | OH.PHAR.247 |
| Denosumab (Prolia, Xgeva) | OH.PHAR.58 |
| Dupilumab (Dupixent) | OH.PHAR.336 |
| Infliximab (Avsola, Inflectra, Remicade, Renflexis, Zymfentra) | OH.PHAR.254 |
| Mepolizumab (Nucala) | OH.PHAR.200 |
| Mirikizumab-mrkz (Omvoh) | OH.PHAR.662 |
| Ocrelizumab (Ocrevus) | OH.PHAR.335 |
| Omalizumab (Xolair) | OH.PHAR.01 |
| Reslizumab (Cinqair) | OH.PHAR.223 |
| Risankizumab-rzaa (Skyrizi) | OH.PHAR.426 |
| Romosozumab-aqqg (Evenity) | OH.PHAR.428 |
| Tezepelumab-ekko (Tezspire) | OH.PHAR.576 |
| Tocilizumab (Actemra, Tofidence, Tyenne) | OH.PHAR.263 |
| Tralokinumab-ldrm (Adbry) | OH.PHAR.577 |
| Ustekinumab (Stelara, Pyzchiva, Selarsdi, Wezlana) | OH.PHAR.264 |
| Vedolizumab (Entyvio) | OH.PHAR.265 |
February 2025
We understand the challenges posed by the recent flu outbreak and recognize the increased strain on hospitals as capacity nears its limits. At Buckeye Health Plan, we are committed to supporting our provider partners and ensuring members receive timely, high-quality care.
While we cannot waive prior authorization requirements at this time, we want to assure you that our processes are designed to balance the need for efficient care delivery with appropriate oversight. Prior authorization helps ensure that members receive medically necessary and appropriate care while also managing healthcare resources effectively.
To facilitate timely hospital discharges and ease transitions to post-acute care, Buckeye currently has Gold Card arrangements with CPAN, OASN and MNS that allow for hospital discharge to these specific facilities without prior authorization. We encourage hospitals to leverage these arrangements to expedite patient flow and optimize care coordination.
Buckeye Health Plan is partnering with the National Council on Independent Living (NCIL) to assist our providers with removing barriers to accessibility at their practice by creating the Barrier Removal Fund (BRF) program.
The goal of BRF is to increase the percentage of participating providers that meet the minimum federal and state disability access standards by providing grant funds. These grant funds go toward providing equal access to quality health care and services that are physically and programmatically inaccessible for our members with disabilities and their companions.
- “Physical access,” also referred to as “architectural access,” refers to a person with a disability’s ability to access buildings, structures, and the environment.
- “Programmatic access” refers to a person with a disability’s access to goods, services, activities and equipment.
The deadline to request these grant funds has been extended to March 14, 2025, at 5:00 p.m. The determination for grant awards will take approximately 4 months.
To apply for these funds, follow these steps:
- Visit the Request for Proposal page of the National Council on Independent Living website.
- Review the Request for Proposal (RFP) document to see if you are eligible.
- If eligible, complete and submit the Request for Proposal PDF online by the stated deadline.
If you have any questions about the Barrier Removal Fund or the RFP, please contact Mary-Kate Wells at mary-kate@ncil.org. We hope your office takes advantage of this important opportunity to assist your patients with disabilities.
Respiratory Syncytial Virus (RSV) Medication Update:
Effective April 1, 2025, Beyfortus (nirsevimab-alip), an FDA approved drug for RSV, will require prior authorization for utilization in the Medicaid population when billed as a medical claim. The relevant CPT codes for Beyfortus are:
- 90380 Respiratory syncytial virus, monoclonal antibody, seasonal dose; 0.5 mL dosage, for intramuscular use
- 90381 Respiratory syncytial virus, monoclonal antibody, seasonal dose; 1 mL dosage, for intramuscular use
As previously communicated, in a continuous effort to make it easier to do business with us, Ambetter from Buckeye Health Plan is introducing Availity Editing Services (AES). Centene is partnering with Availity to return rejection messages on its behalf via AES messages. AES services will now begin March 26, 2025.
Patient Safety Awareness Week - March 9-15, 2025
Join Buckeye Health Plan as we recognize Patient Safety Awareness Week March 9 – 15, 2025. This week highlights the importance of prioritizing patient safety in healthcare systems and the vital role and shared responsibility of the healthcare systems, health organizations, and all medical staff in contributing to patient safety.
Patient Safety Awareness Week is an event designed to stimulate discussions and encourage education on healthcare safety. According to the World Health Organization (WHO), over 2.6 million deaths occur due to unsafe care in hospitals worldwide. Additionally, 40% of patients experience harm in ambulatory and primary care settings, with an estimated 80% of these harms being preventable.
Why is Patient Safety Awareness Week Important?
- It honors patients who were impacted or lost to medical harm and poor healthcare safety.
- It raises awareness to foster greater precaution among healthcare professionals.
- It promotes patient safety education and action.
"Moving the Needle"
This year's theme for Patient Safety Awareness Week is "Moving the Needle" to focus on turning awareness into action for safer patients and care teams. It shifts from merely raising awareness to setting an industry-wide expectation for patient safety action. Healthcare organizations and care team members are encouraged to reflect on questions such as:
- How have I helped to improve patient safety?
- How have I helped to move the needle closer to the safest care possible?
- What am I going to do next to improve care delivery?
Promoting Patient Safety
The principle of "First, do no harm" is fundamental to any healthcare service. Understanding the underlying causes of errors in medical care requires shifting from a traditional blaming approach to system-based thinking. A safe health system should take all necessary measures to avoid harm, including:
- Leadership commitment to safety and promoting a culture of safety.
- Safe procedures and clinical processes.
- Education to promote staff competence, teamwork, and communication.
- Involving patients and families in policy and procedure development.
- Patient safety incident reporting for continuous learning and improvement.
By increasing awareness and taking proactive steps, we can work towards a future where preventable harm is reduced, ensuring your patients and our members receive safe and effective care.
Resources to learn more:
Institute for Healthcare Improvement
Agency for Healthcare Research and Quality
Center for Patient Safety See the Patient Safety Awareness Week Flyer (PDF)
We’d love to hear from you and how you are celebrating Patient Safety Week in your organization. Share your experiences with us by emailing BuckeyeQualityofCare@centene.com – we look forward to hearing from you!
January 2025
In an effort to reduce the administrative burden on providers, the Ohio Department of Medicaid and the MCO’s developed consistent billing guidance for Hospice billing for room and board, and Ventilator services. Highlights include:
- Only accepting HCFA form (CMS-1500) for Hospice Nursing Facility Room and Board (NF R&B)
- Only accepting UB04 form for ventilator and ventilator weaning
- Must include diagnosis code Z99.11 for ventilator and ventilator weaning services (does not have to be primary)
Beginning Jan. 20, 2025, you can validate eligibility and benefits, submit claims, check claim status, submit authorizations and access Buckeye Health Plan payer resources via Availity Essentials.
Availity experts are hosting several live webinar sessions to help you get ready. Here’s a look ahead at what tools and functionality are coming, paired with live training to learn more. Space is limited, save your seat today!
Availity Essentials Introduction
- Tuesday, Jan. 21 – 2:15 p.m. EST
- Monday, Jan. 27 – 3 p.m. EST
- Monday, February 3rd, 3:00 – 4:00 pm EST
Authorization Tools
- Tuesday, Jan. 21 – 4 p.m. EST
- Tuesday, Jan. 28 – 3 p.m. EST
- Tuesday, February 4th, 3:00 – 4:00 pm EST
Claim Submission
- Wednesday, Jan. 22 – 3 p.m. EST
- Wednesday, Jan. 29 – 3 p.m. EST
Claims Follow-up Tools
- Thursday, Jan. 23 – 3 p.m. EST
- Thursday, Jan. 30 – 3 p.m. EST
Risk & Quality Applications
- Thursday, Jan. 23 – 4 p.m. EST
- Wednesday, Jan. 29 – 4 p.m. EST
Please Note: You must be logged into your Availity Essentials account to register and attend live training. To register for an Essentials account, visit Register and Get Started with Availity Essentials.
Enrolling for a provider webinar in the Availity Learning Center (ALC)
- Log in to Availity Essentials.
- Select Help & Training > Get Trained.
- ALC opens in a new browser tab. If it does not, have the user check their browser settings to allow for pop-ups and redirects from apps.availity.com and availitylearning.learnupon.com.
- Select the Sessions tab.
- Select the View Course button next to the webinar.
- Select the Enroll button.
Routine Foot Care Billing
Ambetter reimburses for routine foot care in accordance with state mandates and CMS guidelines. Routine Foot Care services are not restricted to podiatrists. These services may be used by any certified physician or non-physician (NPP) specialty. Based on CMS guide for routine foot care modifiers Q7, Q8 and Q9 are being added to further ensure Ambetter benefit(s) are applying routine foot care cost share for routine foot care services.
All other services will apply Surgical/Specialist/Doctor/Facility cost share depending on provider billing.
Effective 1/1/2025 routine foot care benefit(s) will be updated to align with the CMS guidance provided in Article - Billing and Coding: Routine Foot Care (A57957) (cms.gov).
Prior authorization may be required. Please reference the Provider Resource page.
December 2024
Approximately 500 medical, clinical and laboratory providers have signed agreements with the Ohio Department of Health (ODH) to provide breast and cervical cancer screening and diagnostic services to Breast and Cervical Cancer Project (BCCP) clients. BCCP’s 4 Regional Enrollment Agencies work with medical providers in their respective geographic areas to provide services to BCCP direct service clients.
BCCP direct services include mammograms, Pap tests, office visits, clinical breast exams, colposcopy, breast ultrasound, biopsy and other diagnostic procedures. The CDC determines eligible Current Procedural Terminology codes for BCCP. In general, providers are reimbursed at Medicare Part B rates. Allowable codes and rates are updated annually. Services are reimbursed by a third-party administrator.
Please view this brief video to remind current BCCP providers of the important benefits and opportunities for their patients from the BCCP. If you would like to sign up to be a BCCP provider, you will find the application on the ODH website.
Per CMS Guidance, oral-only End Stage Renal Disease (ESRD) Drugs will become Part D excluded and bundled into the Part B ESRD prospective payment to dialysis clinics effective 01/01/2025. The following drugs will be removed from all Part D formularies starting 01/01/2025.
BRAND NAME | GENERIC NAME |
Phoslyra | Calcium Acetate |
Auryxia | Ferric Citrate |
Fosrenol | Lanthanum Carbonate |
Renvela | Sevelamer Carbonate |
Renagel | Sevelamer HCl |
Velphoro | Sucroferric Oxyhydroxide |
Xphozah | Tenapanor HCl |
The following are high-impact Part-D formulary changes that will affect the Medicare Advantage and MyCare lines of business in 2025.
DRUG REMOVED | DRUG/THERAPEUTIC CLASS | FORMULARY ALTERNATIVE(S) & RESTRICTIONS |
Basaglar KwikPen | Insulin, Long-Acting | Insulin Glargine-YFGN |
Gemtesa | Urinary Antispasmodic | Tolterodine IR/ER [QL]; Solifenacin [QL]; Oxybutynin IR; Oxybutynin ER [QL]; Myrbetriq [QL] |
Fiasp | Insulin, Rapid-Acting | Insulin Aspart |
Pulmicort Flexhaler | Respiratory Inhaler, Steroid | Arnuity Ellipta [QL] |
Levalbuterol HFA | Respiratory Inhaler, SABA | Albuterol Sulfate HFA [QL]; Ventolin HFA [QL] |
Emgality | Migraine, CGRP Inhibitor | Aimovig [PA,QL] |
Silodosin | Benign Prostatic Hyperplasia | Tamsulosin; Alfuzosin ER; Finasteride; Dutasteride [QL] |
Veltassa | Potassium Binder | Sodium Polystyrene Sulfonate; Lokelma |
Fesoterodine ER | Urinary Antispasmodic | Tolterodine IR/ER [QL]; Solifenacin [QL]; Oxybutynin IR; Oxybutynin ER [QL]; Myrbetriq [QL] |
Vyzulta | Glaucoma | Latanoprost; Travoprost; Lumigan |
Simbrinza | Glaucoma | Brimonidine 0.15%, 0.2%; Brinzolamide; Dorzolamide; Dorzolamide-Timolol; Alphagan P 0.1%; Combigan |
Xeljanz, Xeljanz XR | Inflammatory Disorders | Yuflyma [PA,QL]; Cyltezo 40 mg/0.8mL [PA,QL]; Humira [PA,QL] (excluding PDP); Enbrel [PA,QL]; Rinvoq [PA,QL]; Skyrizi [PA,QL]; Stelara [PA,QL] |
Forteo | Osteoporosis | Teriparatide 620 mcg/2.48mL [PA,QL] |
Procrit | Erythroid Stimulant | Retacrit [PA] |
Please note this is not a complete list of formulary changes. Please consult list of drugs change notices for further information.
Buckeye Health Plan is partnering with the National Council on Independent Living (NCIL) to assist our providers with removing barriers to accessibility at their practice by creating the Barrier Removal Fund (BRF) program.
The goal of BRF is to increase the percentage of participating providers that meet the minimum federal and state disability access standards by providing grant funds. These grant funds go toward providing equal access to quality health care and services that are physically and programmatically inaccessible for our members with disabilities and their companions.
- “Physical access,” also referred to as “architectural access,” refers to a person with a disability’s ability to access buildings, structures, and the environment.
- “Programmatic access” refers to a person with a disability’s access to goods, services, activities and equipment.
The deadline to request these grant funds is February 28, 2025, at 5:00 p.m. The determination for grant awards will take approximately 4 months.
To apply for these funds, follow these steps:
- Visit the Request for Proposal page of the National Council on Independent Living website.
- Review the Request for Proposal (RFP) document to see if you are eligible.
- If eligible, complete and submit the Request for Proposal PDF online by the stated deadline.
If you have any questions about the Barrier Removal Fund or the RFP, please contact Mary-Kate Wells at mary-kate@ncil.org. We hope your office takes advantage of this important opportunity to assist your patients with disabilities.
November 2024
Buckeye Health Plan has chosen Availity Essentials as its new, secure provider portal. Starting January 20, 2025 you can validate eligibility and benefits, submit claims, check claim status, submit authorizations, and access Buckeye Health Plans payer resources via Availity Essentials.
If you are already working in Essentials, you can log in to your existing Essentials account to enjoy these benefits for Buckeye’s members beginning January 20, 2025:
- Use Availity Essentials to verify member eligibility and benefits, submit claims, check claim status, currently submit authorizations, and more.
- Look for additional functionality in Buckeye’s payer space on Essentials and use the heart icon to add apps to My Favorites in the top navigation bar. Our current secure portal will still be available for other functions you may use today.
- Access Manage My Organization – Providers to save provider information. You can then auto-populate that information repeatedly to eliminate repetitive data entry and reduce errors.
If you are new to Availity Essentials, getting your Essentials account is the first step toward working with Buckeye Health Plan on Availity.
Getting started: Designate an Availity administrator for your provider organization
Your provider organization’s designated Availity administrator is the person responsible for registering your organization in Essentials and managing user accounts. This person should have legal authority to sign agreements for your organization.
HOW DOES THIS IMPACT ME? | WHAT IS MY NEXT BEST STEP? |
I am the administrator. I am the designated Availity administrator for my organization. | Visit Register and Get Started with Availity Essentials to enroll for training and access other helpful resources. |
I am not the administrator. I am NOT the designated Availity administrator for my organization. | Your designated Availity administrator will determine who needs access to Availity Essentials on behalf of your organization and will add user accounts in Essentials.
|
I am not sure. I am not sure who will be the designated Availity administrator for my organization. | Share this information with your manager to help determine who will be the designated Availity administrator for your organization. |
Check out some of the time-saving tools that come with an Availity Essentials account:
- Verify member eligibility and benefits, submit claims, check claim status, and currently submit authorizations.
- Look for additional functionality in Buckeye’s payer space and use the heart icon to add apps to My Favorites in the top navigation bar.
- Save provider information in Essentials and auto-populate it to save time and prevent errors.
Join one of our upcoming free webinars, Availity Essentials Overview for Buckeye, to learn additional tips for streamlining your workflow. We’ll show you how to verify eligibility and benefits, submit claims, check claim status, submit authorizations, and more.
We're excited to welcome you to Availity Essentials, helping you transform the way you impact patient care with Buckeye Health Plan. If you need additional assistance with your registration, please call Availity Client Services at 1-800-AVAILITY (282-4548). Assistance is available Monday through Friday, 8 a.m. – 8 p.m. ET. For general questions, please reach out to your Buckeye Health Plan Provider Relations Administrator.
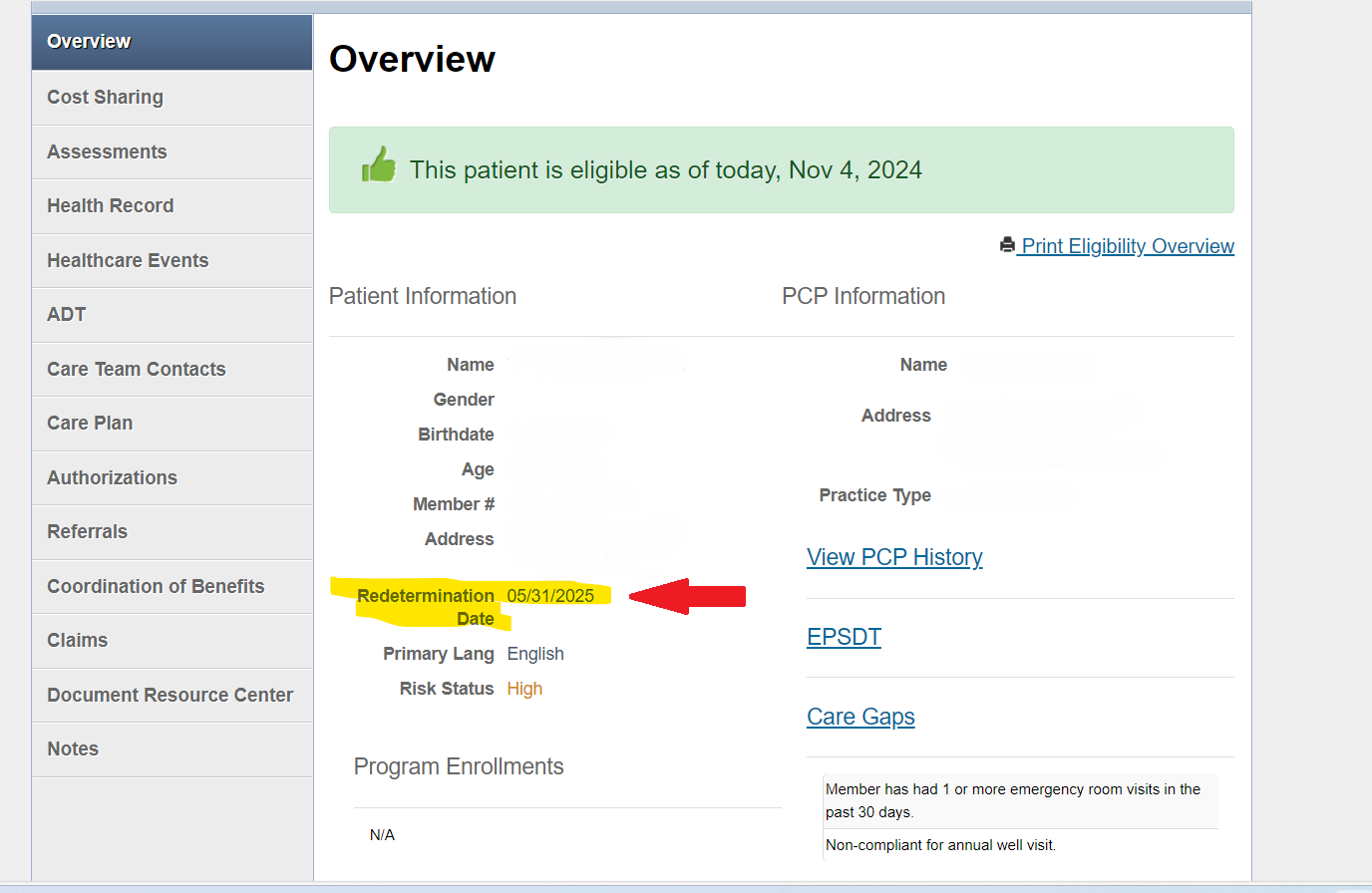
Medicaid members are required to renew their benefits every year to keep their coverage. Buckeye Health Plan conducts outreach directly to our members to remind them when they need to renew and how they can complete their renewal. This process is called Redetermination through the Ohio Department of Medicaid. Members can renew their health care coverage online at www.benefits.ohio.gov, by calling the ODM Consumer Hotline at 1-844-640-6446 or by visiting their local Ohio Department of Job and Family Services (ODJFS) location. To help with this process, we have added each member’s Redetermination Date to Buckeye’s Secure Provider Portal. When you login, you’ll see the member’s Redetermination Date located just below their address on the Overview tab. See the sample image for reference.
In a continuous effort to make it easier to do business with Ambetter from Buckeye Health Plan, we are introducing Availity Editing Services (AES). Starting Jan. 24, 2025, Centene is partnering with Availity to return rejection messages on its behalf via AES messages. These messages will show in your existing workflows. AES will give you an option, but not a requirement, to edit a claim.
AES can identify a claim error upfront and return a message to you for correction before sending the claim on to the plan to be adjudicated.
- You should review Edit Messages for potential corrections to the suggested claim line(s). If you make updates to the claim, this may help the claim process correctly the first time, preventing errors, improving payment accuracy, and claims adjudication turnaround time.
- If, after reviewing the message, you find it does not apply, please resubmit the claim “As-Is” to allow a bypass of the edit in cases where it may not be applicable.
This is not intended as a new method to deny a claim, nor does it bypass or replace downstream edits. If you choose to bypass an edit, it is possible that other downstream edits will still function as normal in our claims systems. Remember to “submit” your claim regardless of your choice to edit or bypass. This action is required for the claim to be processed in our systems.
If you have a Practice Management System (PMS), you can locate your edits report within your claims workbasket or que reporting. If you submit claims via the Availity portal, any of these rejections will show on your normal reports.
If you need assistance with registering for Availity Essentials, please call Availity Client Services at 1-800-AVAILITY (282-4548). Assistance is available Monday through Friday, 8 a.m. – 8 p.m. ET.
If you choose to submit claims via Availity, join one of Availity’s free webinars to learn additional tips for streamlining your workflow:
- Send and Receive EDI Files – Training Demo
This demo shows users where/how they can access reports in Availity Essentials. On these reports are where they would see edits. Please note: this demo does not say/call it AES however, this is the demo that would show the user how to locate the reports. - EDI Reporting Preferences – Training Demo
This demo shows users how to setup their EDI Reporting Preferences which needs to be done first by the user’s organization’s Availity Administrator to access the reports in the Send and Receive EDI Files application.
For general questions, please reach out to your Ambetter from Buckeye Health Plan Provider Engagement Administrator.
As of September 30, 2024, there is a change in the way Medicare covers antiretroviral therapy that is approved by the U.S. Food and Drug Administration (FDA) to prevent human immunodeficiency virus (HIV) infection in individuals at high risk of acquiring HIV. Use of these medications to prevent HIV infection is known as pre-exposure prophylaxis (PrEP).
Based on new Medicare coverage rules, medications prescribed for PrEP will be covered under Medicare Part B without cost sharing (i.e., deductibles or copays) as an additional preventive service. PrEP medications will no longer be covered under the Medicare Part D benefit. Medications for HIV treatment and HIV post-exposure prophylaxis (PEP) will remain coverable under Medicare Part D.
The table below lists the medications that are FDA-approved to be used as PrEP for HIV prevention. Please note that some of these medications are also FDA-approved to be used for HIV treatment.
As of September 30, 2024, Apretude will no longer be covered under Medicare Part D since it is only FDA-approved for PrEP for HIV prevention. In addition, Descovy, Truvada, and generics for Truvada will be covered only under Medicare Part B when used for PrEP for HIV prevention but will remain covered under Medicare Part D when used for HIV treatment.A diagnosis code should be included on all prescriptions if you are prescribing one of these medications for PrEP for HIV prevention.
Brand Drug Name | Generic Drug Name | FDA-Approved Use |
Apretude® | cabotegravir | PrEP for HIV prevention |
Descovy® | emtricitabine and tenofovir alafenamide | PrEP for HIV prevention HIV treatment |
Truvada® | emtricitabine and tenofovir disoproxil fumarate | PrEP for HIV prevention HIV treatment |
emtricitabine and tenofovir disoproxil fumarate | emtricitabine and tenofovir disoproxil fumarate | PrEP for HIV prevention HIV treatment |
As a valued member of the Buckeye Health Plan network, we are providing this important notification for you.
Ohio Department of Medicaid (ODM) notified Buckeye Health Plan some 835 files from June of 2024 were not received by ODM and therefore some providers may not have received necessary remits. If you were impacted and have not received every remit, those remits can be retrieved by logging into Payspan.
If you should have further questions, please reach out to Provider Services, Monday through Friday 7am to 8pm at 866-296-8731.
Thank you again for being a valued provider for our members, your patients.
October 2024
A New Program That Makes Rx Drugs More Affordable by Allowing Medicare Members to Spread Their Prescription Costs Over Time
Passed into law August 2022 by President Biden, H.R. 5376 — Inflation Reduction Act (IRA) includes policies on Medicare drug pricing. The IRA significantly reforms the Medicare Part D benefit design, including a new program, Medicare Prescription Payment Plan (M3P), which will be available to all eligible Medicare members1, beginning Jan. 1, 2025.
Program Overview for Eligible Participating Medicare Members1
- Financial benefits to all Medicare members1 in 2025 include an elimination of the coverage gap and capping the maximum out-of-pocket (OOP) prescription costs at $2,000 annually — which beneficiaries can spread across the plan year.
- M3P participants will pay $0 at the pharmacy for covered Part D drugs and be billed monthly for any cost-sharing they incur while in the program. Importantly, this will help them manage prescription costs by enabling them to spread their monthly payments over time.
- Payment might change every month as additional prescriptions are filled.
- The program is voluntary, and eligible members can choose to opt-in to the program during the annual enrollment period and throughout the plan year. Members can conveniently opt-in via online, by phone, or mail.
o Online: express-scripts.com/mppp
o Phone: 833-750-9969
o Mail:
Mail Express Scripts Medicare
Prescription Payment Plan
P.O. Box 2
St. Louis, MO 63166
- Existing members will receive additional information in their Annual Notice of Change.
- New members will receive additional information within 10 days of confirmed enrollment.
1Excludes plans that solely charge $0 cost sharing for Part D covered drugs. See your plan’s Evidence of Coverage for more details.
Questions or Concerns?
As always, we encourage you to use the resources on Medicare.gov/prescription-payment-plan or contact your Provider Services team.
September 2024
Earlier this month Buckeye Health Plan joined the World Health Organization (WHO) in recognizing and creating awareness of World Patient Safety Day. This day is as an opportunity to raise public awareness and foster collaboration between patients, health workers, policymakers and health care leaders to improve patient safety.
This year, the theme is “Improving diagnosis for patient safety” with the slogan “Get it right, make it safe!”, highlighting the critical importance of correct and timely diagnosis in ensuring patient safety and improving health outcomes.
According to the World Health Organization, diagnostic errors account for 16% of preventable patient harm and are common in all health care settings. These can include missed, incorrect, delayed, or miscommunicated diagnoses and can have significant consequences.
We encourage you to visit the WHO Patient Safety Day to find the latest research, resources, and suggested diagnostic interventions to help improve diagnostic safety.
Additional great resources on Patient Safety and diagnostic safety can also be found from the Agency for Healthcare Research and Quality:
Toolkit for Engaging Patients to Improve Diagnostic Safety and
Team Strategies and Tools to Enhance Performance & Patient Safety (TeamSTEPPS)
Thank you for helping ensure the health and safety of our communities.
Does the two-midnight benchmark apply to Medicare Advantage plans?
Yes. Medicare Advantage plans must provide coverage and pay for an inpatient admission when, based on consideration of complex medical factors (e.g., history and comorbidities, the severity of signs and symptoms, current medical needs, the risk of an adverse event occurring during hospitalization) documented in the medical record, the admitting physician expects the patient to require hospital care that crosses two midnights.1 Medicare Advantage Plans may still use prior authorization or concurrent case management review to determine if the complex medical factors are sufficiently documented in the medical record to support medical necessity of the inpatient admission.2
Does the two-midnight rule presumption apply to Medicare Advantage plans?
No. The presumption that all inpatient claims that cross two midnights are appropriate for payment and therefore should not be the focus of medical review does not apply to Medicare Advantage Plans. The two-midnight presumption directs medical reviewers to select Original Fee-for-Service Medicare Part A claims for review under a presumption that hospital stays that span two midnights after an inpatient admission are reasonable and necessary Part A payment.3
Does Wellcare by Allwell utilize medical necessity criteria?
Yes. Medicare Advantage plans such as Wellcare may apply internal coverage criteria when coverage criteria is not fully established in Medicare laws, national coverage determinations and/or local coverage determinations. As such, Medicare Advantage plans are permitted to create their own internal coverage criteria based upon widely-used treatment guidelines or clinical criteria, and may use coverage criteria products such as InterQual® or the like.4
Is the two-midnight benchmark enough to qualify an inpatient admission for coverage?
No. Wellcare by Allwell reviews inpatient admissions within the context of the patient’s medical record to evaluate whether the patient’s documented complex medical factors support hospital care that exceeds two midnights. As stated above, Wellcare by Allwell reviewers may consider other coverage criteria such as InterQual when conducting medical necessity reviews.
Can stays less than 24 hours qualify for inpatient admission payment?
In the majority of cases, hospital stays less than 24 hours do not meet criteria for payment as an inpatient stay. However, hospital services that do not exceed two midnights may be medically necessary in certain cases, such as an unexpected death, patient departure against medical advice, election of hospice in lieu of continued treatment in a hospital, and for a procedure on the CMS Inpatient Only List.
Does Wellcare by Allwell follow the case-by-case exception?
Yes. Generally, medical necessity will be met if an admitting physician does not expect a patient to require hospital care exceeding two midnights, but determines, based on complex medical factors documented in the medical record, that inpatient care is nonetheless necessary.5
1. 88 Fed.Reg. 222120, 22191 (April 12, 2023)
2. Id. at 22192
3. Id
4. Id. at 22194-22195
5. Id. at 22191
August 2024
Per Gainwell Technologies, the Medicaid Single Pharmacy Benefit Manager:
Effective 9/1/2024, the following medications will require prior authorization for Medicaid Managed Care Plan members if there is not already an active PA on file:
- Bydureon Bcise (exenatide)
- Mounjaro (tirzepatide)
- Ozempic (semaglutide)
- Rybelsus (semaglutide)
A letter has been sent out to members that may be affected by this policy.
Prescribers can submit a prior authorization electronically, by faxing Gainwell Pharmacy Services at 833-679-5491, or by calling 833-491-0344 (TTY 833-655-2437). Submissions for these medications will be reviewed according to the Ohio Medicaid Unified Preferred Drug List (UPDL) clinical criteria.
For more details, visit SPBM
1. Select “Reference Material”
2. Then “Unified Preferred Drug List”
3. And view “UPDL criteria effective 07.01.2024”
As part of our all-MCO CPT II Workgroup initiative, we would like to announce an upcoming virtual training offering presented by ArchProCoding. This is an EMR agnostic beginning-level training, meant to identify best practices related to how clinical providers accurately document their care and diagnoses. We are also excited to announce that there will be an opportunity for CEUs provided with this training. This 90-minute training will be offered on two different dates:
- September 24 (10:00 a.m.)
- September 25 (2:00 p.m.)
If you have questions or would like to be added to the communication list for this training, please email MHOVBR@molinahealthcare.com. We hope to see you there!
Procedure Code | Procedure Code Description |
New Standard |
Line of Business |
97110 | THERAPEUTIC EXERCISES | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97010 | HOT OR COLD PACKS THERAPY | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97140 | MANUAL THERAPY 1/> REGIONS | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97113 | AQUATIC THERAPY/EXERCISES | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97112 | THERAP PROC 1/> AREAS EA 15 MIN; BALANCE/COORDIN | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97039 | PHYSICAL THERAPY TREATMENT | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97530 | THERAPEUTIC ACTIVITIES | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
97533 | SENSORY INTEGRATION | Pre-authorization is required after 8 Physical or Occupational Therapy visits for All Providers | Medicaid |
G0151 | SRVC PT HOM HLTH/HOSPICE EA 15 MIN | Pre-authorization is required for All Providers | Medicaid |
G0152 | SRVC OT HOM HLTH/HOSPICE EA 15 MIN | Pre-authorization is required for All Providers | Medicaid |
G0153 | SRVC SPCHANDLANG PATH HH/HOSPIC EA 15 | Pre-authorization is required for All Providers | Medicaid |
E1390 | OXY CNCNTRTR/1 DLVRY PORT/CPBLE OF DLVRNG 85%OR>OXY CNCNTRTN | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
E1391 | OXY CNCNTRTR/DUAL DLVRY PRT/CPBL DLVRNG 85%OR>OXY CNCNTRTN | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
E1392 | PORTABLE OXYGEN CONCENTRATOR | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
E0470 | RSPRTRY DVCE/BI-LVL PRESS CPLTY/WOUT BCKP RATE FTRE/W NNINVSV INTRFC | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
E0471 | RSPRTRY DVCE/BI-LVL PRESS CPLTY/W BCKP RATE FTRE/W NNINVSV INTRFC | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
E0601 | CONTINUOUS AIRWAY PRESSURE (CPAP) DEVICE | Pre-authorization is required after 30 days from start of service date for ALL Providers | Medicaid |
In preparation for expanded coverage for Doula Services under Ohio Medicaid, Buckeye is reaching out to our partner practices and medical groups, and independent practitioners in the Doula Community with an interest in providing these services for our members. ODM is hosting a series of training sessions for doulas starting in September and registration for that training series can be found on the ODM doula webpage. Additional details are shown in the article below.
Over the coming months, Buckeye will also be conducting training and rolling out resources and tools to onboard our new partners in care. If you are interested in learning more, or would like to be included in upcoming trainings, please contact Mindy Ridgeway at melinda.ridgeway@centene.com. Include in the subject line, "Doula Resources"
Upcoming Doula Trainings
The Ohio Department of Medicaid (ODM), along with our partners at the Ohio Board of Nursing (BON) and Medicaid Managed Care Organizations (MCO), will be hosting a training series for doulas. We hope that anyone interested in becoming a certified doula in the State of Ohio with the intent to serve Ohio Medicaid members, will register for this training series. Each virtual training session date and time, along with a quick snapshot of the agenda, can be found below. Registration for the training series can be found on the ODM doula webpage.
- Training session 1: Tuesday, September 10 from 1:30 p.m.-3:30 p.m.
- Medicaid 101, National Provider Identifier (NPI) basics, BON certification process, ODM provider enrollment process
- Training session 2: Thursday, September 26 from 10 a.m.-12 p.m.
- MCO 101 and contracting process
- Training session 3: Tuesday, October 29 from 1:30 p.m.-3:30 p.m.
- MCO claims billing and prior authorization process
- Training session 4: Thursday, November 7 from 10 a.m.-12 p.m.
- Fee-for-service billing and prior authorization process
- Training session 5: Thursday, November 21 from 12:30 p.m.-2 p.m.
- Related maternal health topics overview
We are pleased to announce that we recently added a new form on our website to allow providers to indicate on their profile that they offer Telehealth services. See our Telehealth section to learn where to find the form and tips from ODM on how to Announce Telehealth Availability.
July 2024
Our annual provider satisfaction survey will launch later this summer and we hope you’ll take a moment to share your feedback. This survey serves as the foundation for key improvement initiatives that we undertake each year and your feedback is critical to making sure we address the issues that are important to you. Last year, a few of the initiatives we accomplished based on your feedback are:
- Webpage dedicated to finding your Provider Engagement Administrator. That way you can always find your advocate.
- Enhanced ability to track authorization status online.
- Initiated the Care Guide program to assist engaging with your patients and closing care caps.
- Increased number of high quality network specialists to enhance your referral options.
We look forward to learning about how we can continue to improve your experience doing business with us. Please keep an eye out for our survey in the coming weeks.
The Centers for Medicare & Medicaid Services (CMS) requires health plans to provide annual education and training on our Special Need’s Plans (SNP) Model of Care to providers who treat our SNP members. This applies to our Dual Eligible Special Needs Plan (D-SNP) members, who are eligible for both Medicare and Medicaid, and our Chronic Condition Special Needs Plan (C-SNP) members.
As stated in the Provider Manual, all providers who treat our SNP members regardless of network participation status must complete Model of Care (MOC) training annually by December 31st of each year.
The training is designed to help you better understand our approach to the delivery of care for SNP members.
See our Required Training Section of the website and click on MOC.
June 2024
Our annual provider satisfaction survey will launch later this summer and we hope you’ll take a moment to share your feedback. This survey serves as the foundation for key improvement initiatives that we undertake each year and your feedback is critical to making sure we address the issues that are important to you. Last year, a few of the initiatives we accomplished based on your feedback are:
- Webpage dedicated to finding your Provider Engagement Administrator. That way you can always find your advocate.
- Enhanced ability to track authorization status online.
- Initiated the Care Guide program to assist engaging with your patients and closing care caps.
- Increased number of high quality network specialists to enhance your referral options.
We look forward to learning about how we can continue to improve your experience doing business with us. Please keep an eye out for our survey in the coming weeks.
Payment Policies Effective 8-1-24
| Policy Name | Policy Revision Date | More or Less Restrictive | Configuration Updates and Reason | Applicable Lines of Business |
| GI Pathogen Nucleic Acid Detection Panel Testing (CP.MP.209) | 3/23 | More and Less | Applied POS and diagnosis code edits to 87506; added new payable POS for 87506 and 87507; added coding combinations for diagnosis code matching as payable with 87506 and 87507, all consistent with the CMS Local Coverage Determination the policy is based on. | AM Medicaid and AM Marketplace |
| Wheelchair Seating (CP.MP.99) | 1/24 | More and Less | Edits made to align with recent changes made to the CMS local coverage article the policy is based upon, including new-for-2024 ICD-10 codes. | AM Medicaid and AM Marketplace |
| ADHD (CP.BH.124) | 2/23 | More | Changes made per literature review, payer comparison and specialist consultation. Internal BH Psy.Ds. reviewed and approved the following changes, as did an external specialist: Neuropsychological testing codes removed from edit (now made payable with only an ADHD diagnosis on the claim): 96132 and 96133. The following codes were added as not medically necessary/not payable when billed only with a diagnosis of ADHD on the claim: • 70496- CT angiography, head, with contrast • 70554-Magnetic resonance imaging, brain, functional MRI; including test selection and administration of repetitive body part movement and/or visual stimulation, not requiring physician or psychologist administration • 70555-Magnetic resonance imaging, brain, functional MRI; requiring physician or psychologist administration of entire neurofunctional testing • 78610-Brain imaging, vascular flow only • 84436-Thyroxine; total • 84437-Thyroxine; requiring elution (eg, neonatal) • 84439-Thyroxine; free • 84442-Thyroxine binding globulin (TBG) • 84443-Thyroid stimulating hormone (TSH) • 84445-Thyroid stimulating immune globulins (TSI) • 84478-Triglycerides • 84479-Thyroid hormone (T3 or T4) uptake or thyroid hormone binding ratio (THBR) • 84481-Triiodothyronine T3; free • 92568-Acoustic reflex testing, threshold • 92569-Acoustic reflex testing; decay • 92570-Acoustic immittance testing, includes tympanometry (impedance testing), acoustic reflex threshold testing, and acoustic reflex decay testing • 95954-Pharmacological or physical activation requiring physician or other qualified health care professional attendance during EEG recording of activation phase (eg, thiopental activation test) • 96020-Neurofunctional testing selection and administration during noninvasive imaging functional brain mapping, with test administered entirely by a physician or other qualified health care professional (ie, psychologist), with review of test results and report • 96902-Microscopic examination of hairs plucked or clipped by the examiner (excluding hair collected by the patient) to determine telogen and anagen counts, or structural hair shaft abnormality • 97010-Application of a modality to 1 or more areas; hot or cold packs • 97012-Application of a modality to 1 or more areas; traction, mechanical • 97014-Application of a modality to 1 or more areas; electrical stimulation (unattended) • 97016-Application of a modality to 1 or more areas; vasopneumatic devices • 97018-Application of a modality to 1 or more areas; paraffin bath • 97022-Application of a modality to 1 or more areas; whirlpool • 97024-Application of a modality to 1 or more areas; diathermy (eg, microwave) • 97026-Application of a modality to 1 or more areas; infrared • 97028-Application of a modality to 1 or more areas; ultraviolet • 97032-Application of a modality to 1 or more areas; electrical stimulation (manual), each 15 minutes • 97033-Application of a modality to 1 or more areas; iontophoresis, each 15 minutes • 97034-Application of a modality to 1 or more areas; contrast baths, each 15 minutes • 97035-Application of a modality to 1 or more areas; ultrasound, each 15 minutes • 97036-Application of a modality to 1 or more areas; Hubbard tank, each 15 minutes | AM Medicaid and AM Marketplace and AM Medicare |
| Laser Therapy for Skin Conditions (CP.MP.123) | 3/23 | Less | Added medically necessary diagnosis codes as payable to match newly added indications (per NCCN guidelines) for cutaneous T-cell lymphoma | AM Medicaid and AM Marketplace |
| Testing for Select Genitourinary Conditions (CP.MP.97) | 9/23 | Less | Edits made after payer comparison, literature review and consultation with internal OB, Dr. Steiner: Changed age at which any payment edit restrictions apply to 16 years and older, as this represents the upper limit of menarche, after which the diagnosis restrictions in the policy would be appropriate (previously the edits applied to ages 13 and over; the revision is more lenient). 81513, 81514, 0352U and 87511 as payable when billed with vaginitis codes in Table 2; added UTI in pregnancy dx codes to Table 2 (med nec codes). Added additional dx codes for asymptomatic screening to not med nec dx code table. 0353U removed from all payment edits; not in scope for policy. Removed vaginitis-related diagnosis codes from Table 7, to allow payment for candida amplified probe testing with vaginitis symptoms. | AM Medicaid and AM Marketplace |
| Urodynamic Testing (CP.MP.98) | 3/23 | Less | Changed N40.3 Nodular prostate with lower urinary tract symptoms from med nec only when billed with 51798 to med nec when billed with any procedure code in policy, per the CMS Local Coverage Determination on urodynamic testing. | AM Medicaid and AM Marketplace and AM Medicare |
| Measurement of Serum 1,25-dihydroxyvitamin D (CP.MP.152) | 9/23 | Less | Add the following ICD-10 codes as payable when billed with CPT code 82652, in addition to those already payable: E89.2- Postprocedural hypoparathyroidism added per literature review (The Journal of Clinical Endocrinology & Metabolism. Serum 1,25-Dihydroxyvitamin D as a Biomarker of the Absence of Hypercalciuria in Postsurgical Hypoparathyroidism) and external specialist recommendation. M83.8 and M83.9- Adult osteomalacia codes added to align with indication for oncogenic osteomalacia (previously only cancer codes were included for this indication) | AM Medicaid and AM Marketplace |
| Holter Monitors (CP.MP.113) | 9/23 | Less | Added new for 2023 and new for 2024 ICD-10 codes as payable with holter monitors: The following were added as medically necessary to align with the indication for assessment of suspected variant angina in the policy: I25.112 Atherosclerosic heart disease of native coronary artery with refractory angina pectoris I25.702 Atherosclerosis of coronary artery bypass graft(s), unspecified, with refractory angina pectoris I25.712 Atherosclerosis of autologous vein coronary artery bypass graft(s) with refractory angina pectoris I25.722 Atherosclerosis of autologous artery coronary artery bypass graft(s) with refractory angina pectoris I25.732 Atherosclerosis of nonautologous biological coronary artery bypass graft(s) with refractory angina pectoris I25.752 Atherosclerosis of native coronary artery of transplanted heart with refractory angina pectoris I25.762 Atherosclerosis of bypass graft of coronary artery of transplanted heart with refractory angina pectoris I25.792 Atherosclerosis of other coronary artery bypass graft(s) with refractory angina pectoris The following codes were added as payable within existing medically necessary code ranges in the policy: Angina pectoris code range: I20.2 I20.81 I20.89 Nonrheumatic mitral valve disorders code range: I34.81 I34.89 Paroxysmal tachycardia code range: I47.10 I47.11 I47.19 I47.20 I47.21 I47.29 | AM Medicaid and AM Marketplace |
| Digital EEG Spike Analysis (CP.MP.105) | 1/24 | Less | New 2024 ICD-10 codes for intractable epilepsy added consistent with existing policy stance: G40.C11 Lafora progressive myoclonus epilepsy, intractable, with status epilepticus G40.C19 Lafora progressive myoclonus epilepsy, intractable, without status epilepticus | AM Medicaid and AM Marketplace |
| EEG for headache (CP.MP.155) | 12/23 | More | Updated not medically necessary migraine code range to reflect new for 2024 ICD-10 codes- changed from G43.001-G43.919 to G43.001-G43.E19. | AM Medicaid and AM Marketplace |
| Pulmonary Function Testing (CP.MP.242) | 12/23 | Less | Added new for 2024 ICD-10 codes as payable for all PFTs in the scope of the policy except bronchoprovocation testing: J15.61 Pneumonia due to Acinetobacter baumannii J15.69 Pneumonia due to other gram-negative bacteria J44.81 Bronchiolitis obliterans and bronchiolitis obliterans syndrome J44.89 Other specified chronic obstructive pulmonary disease J4A.0 Restrictive allograft syndrome J4A.8 Other chronic lung allograft dysfunction J4A.9 Chronic lung allograft dysfunction, unspecified Note: per UpToDate, bronchoprovocation testing is mainly intended for diagnosing asthma, for cough and testing for reaction to environmental exposures. None of the above new codes fall in these categories. | AM Medicaid and AM Marketplace |
| Scanning Computerized Ophthalmic Diagnostic Imaging (CP.VP.14) | 11/23 | Less | Updated medically indicated diagnoses for posterior segment, retina 92134 – SCODI to include new ICD-10 codes for sickle-cell retinopathy. | AM Medicaid and AM Marketplace and AM Medicare and XL Medicare |
| Extended Ophthalmoscopy (CP.VP.26) | 11/23 | Less | Updated medically indicated diagnoses to include new ICD-10 codes for sickle-cell retinopathy. | AM Medicaid and AM Marketplace and AM Medicare and XL Medicare |
| Fluorescein Angiography (CP.VP.28) | 11/23 | Less | Updated medically indicated diagnoses to include new ICD-10 codes for sickle-cell retinopathy. | AM Medicaid and AM Marketplace and AM Medicare and XL Medicare |
| Fundus Photography (CP.VP.29) | 11/23 | Less | Updated medically indicated diagnoses to include new ICD-10 codes for sickle-cell retinopathy. | AM Medicaid and AM Marketplace |
| Gonioscopy (CP.VP.31) | 11/23 | Less | Updated medically indicated diagnoses to include new ICD-10 codes for sickle-cell retinopathy. | AM Medicaid and AM Marketplace |
| Visual Field Testing (CP.VP.63) | 11/23 | Less | Updated medically indicated diagnoses to include new ICD-10 codes for sickle cell retinopathy and chronic migraine with aura. | AM Medicaid and AM Marketplace and AM Medicare and XL Medicare |
CPT Category II Codes are intended to facilitate the collection of information about the quality of care delivered by coding several services or test results that support performance measures.
By submitting CPT Category II and HCPCS codes you are able to report services and/or values based on nationally recognized, evidence-based performance guidelines for improving quality of care for patients/members, benefits including:
- Decrease the need for chart abstractions
- Better reporting of open and closed care needs for your assigned members
- Increase in Payment for Quality (P4Q) due to submission of additional codes
- Gap closure is reported timelier with code submission versus medical records
- Can improve member outcomes and the health of patients
WellCare by Allwell has taken steps to ensure submissions pass through clearing house without issue for select CPT II and HCPCS codes to the Medicare fee schedule at a price of $0.01. This will result in fewer dropped codes by billing companies due to non-payable codes. Review the flyer to learn more details about codes included in this program
Our Medicare Advantage* and Prescription Drug Plans offer a robust, dependable pharmacy network with over 60,000 pharmacies in network — many offering preferred cost-sharing! See the 2024 Benefit Updates.
CenTeam’s very own Chief Health Officer, Dr. Alice Chen, has been named to Modern Healthcare’s 50 Most Influential Clinical Executives list for the second year in a row. Dr. Chen’s commitment to advancing Centene’s health equity and drivers of health efforts, including spearheading the REAL (race, ethnicity and language) SOGI (sexual orientation, gender identity) DOH (drivers of health) Data Project, was a cornerstone to her nomination. Dr. Chen’s focus on health equity and drivers of health has been a top priority for her throughout her career. Learn more about her passion for this critically important work and CenTeam’s commitment to advancing health equity.
Provider Advisory Committee – Interested?
Are you interested in being part of the change you would like to see in healthcare? Buckeye Health Plan is looking for practitioners to participate in our Provider Advisory Committee (PAC) as an advocate for providing the best service to our patients and community.
As a member of the PAC, you are the voice of the members and the provider partners of every specialty.
You can share firsthand what our members need, what is important to them and how we as a health plan can best provide that service.
Our PAC meets four times a year for one hour to share your knowledge and expertise in the delivery of care to improve our members’ well-being through healthy behavior, disease prevention, and self- management of chronic conditions. Other PAC participants include Buckeye Health Plan Medical Directors, network development staff, medical affairs, Ohio Department of Medicaid Representatives, and guest speakers. Topics of discussion include new initiatives, programs, counsel-directed issues, and community resources.
Below are a few examples of topics discussed in past PAC meetings:
- Provider Advisory Roles/Goals, Medicaid in Ohio, Population Health Strategy
- Pharmacy and Prior Authorizations - What is the purpose and how can we make it make it easier for high quality providers?
- Annual Provider Satisfaction Survey Results - How do health plans use the surveys and improve provider satisfaction?
- New Sleep Treatment Pilot - Buckeye partnered with providers to offer a solution for Sleep Apnea.
- BHP Diamond Designation Program - The council asked about ways that health plans recognize and reward high quality providers.
- BHP Alternative Payment Model (APM) Strategy 2023 – 2027- Continued conversation from Senior Leaders about rewarding high quality providers.
- Buckeye Health Plan SDoH Care Coordination Mode l - The council wanted to learn how a health plan could help their patients if they identified non-clinical barriers to care at the site of health care.
If you interested in joining the Provider Advisory Committee, please notify us via email at: buckeyerequests@centene.com. We look forward to hearing from you.
May 2024
Buckeye Health Plan is streamlining the process for Medicaid provider faxed requests for urine drug screen testing. Beginning in June, please fax all requests for urine drug screen testing, both Behavioral Health related testing requests and Physical Health related testing requests to 866-535-4083. Providers should use the Medicaid Outpatient Prior Authorization Form (PDF) For your convenience, the above fax number is located on the form for ease of use.
Buckeye Health Plan is committed to providing an individual approach when reviewing requests for home health services, including Private Duty Nursing services (PDN). We do not apply hard limits on the number of days in a Medicaid certification period when members with special, complex needs are identified, and those needs are not expected to change over an extended period of time. Providers may request a longer certification period for these authorizations with supporting clinical documentation.
April 2024
Additional Prior Authorization Requirements
Service Code | Service/Procedure Description | Comments |
95810 | POLYSOMNOGRAPHY; AGE 6 YEARS OR OLDER, SLEEP STAGING WITH 4 OR MORE ADDITIONAL PARAMETERS OF SLEEP, ATTENDED BY A TECHNOLOGIST |
|
95811 | POLYSOMNOGRAPHY; AGE 6 YEARS OR OLDER, SLEEP STAGING WITH 4 OR MORE ADDITIONAL PARAMETERS OF SLEEP, WITH INITIATION OF CPAP THERAPY OR BILEVEL VENTILATION ATTENDED BY A TECHNOLOGIST |
|
In response to your feedback, we have removed 16 services from our prior authorization list for the Medicaid line of business effective June 1, 2024, for contracted providers, Prior authorization will be required for Non-Contracted Providers, except where indicated:
Service Code | Service/Procedure Description | Comments |
80307 | DRUG TEST PRSMV CHEM ANLYZR |
|
C9257 | INJECTION BEVACIZUMAB 0.25 MG |
|
99499 | UNLISTED EANDM SERVICE |
|
L0639 | LSO SAG-COR CNTRL RIGD SHELL PREFAB |
|
81200 | ASPA GENE |
|
81329 | SMN1 GENE ANALYSIS DOSAGE/DELET ALYS W/SMN2 ALYS | No PA for All Providers |
81243 | FMR1 GENE DETECTION |
|
81256 | HFE GENE |
|
E2402 | NEG PRSS WND TX PUMP STATN/PRTBL |
|
81220 | CFTR GENE COM VARIANTS | No PA for All Providers |
J3489 | INJECTION ZOLEDRONIC ACID 1 MG |
|
S8424 | GRADENT PRESS AID SLEEVE READY MADE | |
E0784 | EXTERNAL AMB INFUSION PUMP INSULIN | |
E0619 | APNEA MONITOR W/RECORDING FEATURE |
|
J0185 | INJECTION APREPITANT 1 MG |
|
E0303 | HOS BED HEVY DUTY WT CAP >350<=600 |
|
J1453 | INJECTION FOSAPREPITANT 1 MG |
|
E0260 | HOS BED SEMI-ELEC W/RAIL W/MATTRSS |
|
J0640 | INJ LEUCOVORIN CALCIUM PER 50 MG |
|
20550 | INJ TENDON SHEATH/LIGAMENT |
|
See the 3-28 Update Below for Full Details
Beginning June 1, 2024, Buckeye will expand the requirements for billing of genetic and molecular testing. In accordance with the Reimbursement Policy for Genetic/Molecular Test Coding Policy, all providers billing for genetic and molecular testing services will be required to adhere to the coding recommendation in the Concert Genetics portal. The policies are posted on our Buckeye Health Plan Policies Website Page for your review.
| Policy Number | Policy Title | Date of Revision | Plan Implementation Date | All Products |
| CG.CC.PP.01 | Lab Testing Payment Policy V1.2024 | 11/8/2023 | 6/1/2024 | X |
| CG.CP.MP.01 | ID Respiratory Lab Tstg V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.02 | ID Multisystem Lab Tstg V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.03 | Dermatologic Lab Tstg V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.04 | Gastroenterologic Lab Tstg Policy V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.05 | Primary Care Preventive Lab Screening V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.06 | ID Vector Borne_Tropical Disease Tstg V1.2024 | 3/1/2024 | 6/1/2024 | X |
| CG.CP.MP.07 | ID Genitourinary Lab Tstg V1.2024 | 3/1/2024 | 6/1/2024 | X |
Thank you for your continued partnership with Buckeye Health Plan. Buckeye is committed to continuously evaluating and improving overall Payment Integrity solutions, as required by State and Federal governing entities. We are providing notice of an additional review that will go into place on or after June 1, 2024
WellCare claims will be reviewed for coordination of benefits by Rawlings, an external vendor. In accordance with policy CC.PI.09 (Coordination of Benefits/Third Party Liability/Subrogation), providers will begin seeing correspondence from Rawlings when these claims are identified as being incorrectly paid for members with other primary coverage. Instructions for next steps and how to reach out for additional information will be included in the letters from Rawlings.
Helping you care for your patients is our top priority. Strong communication and trust between you and your patients will help ensure they’re satisfied and have good outcomes. You can rely on Buckeye Health Plan for information and support to help you keep those patient relationships strong. See our latest notification.
With the goal of improving maternal and infant outcomes for members across the state and in support of our partners in care, Buckeye is pleased to announce a policy update, coming June 1, 2024
Background
Aligning with the recent obstetric payment policy statements from the American Academy of Obstetrics and Gynecology (ACOG), Buckeye announces the release of Professional Claims Guidelines for Obstetrical Billing and Coding. These guidelines will become effective June 1, 2024.
In their statement, released in March, ACOG announced the shift from endorsement of bundled obstetrical payments, to support of methodologies that accurately collect and report the complexity of care from the first prenatal visit through delivery and postpartum.
"Current payment methodologies, especially those using the bundled global maternity codes for obstetric services, no longer reflect the care occurring today…. Those payers that have strictly used the global maternity codes do not have large-scale administrative data regarding the number of visits, the medical decision-making for each visit, or the time and complexity of labor management. This has left a dearth of data available for appropriate, accurate risk adjustment calculations needed to develop alternative payment models."
ACOG Clinical Information - 2024 Payment for obstetricians and gynecologists.
Unbundled Obstetrical Billing
CPT Codes: 59409; 59514; 59612; 59620
Providers are advised to itemize (unbundle) claims for antepartum care, delivery only and postpartum care. Each service should be billed independently of one another. Reporting service in this manner is consistent with ACOG’s advisement, allowing for accurate and complete reporting each service and complexity of care.
New – Expanded Postpartum Visit Coverage
CPT Code: 59430
- Providers may be reimbursed for up to three (3) outpatient postpartum visits complete between day 7-84 postpartum.
- The Postpartum Visits should be reported with CPT 59430 following the report of a Delivery Only CPT Code.
Bundled Obstetrical Billing
CPT Codes: 59410; 59515; 59622
Providers may submit Bundled Obstetric Codes.
- The claim for the Bundled Obstetric service (delivery and postpartum) must include both the Delivery CPT Code and the outpatient postpartum visit reported using CPT II 0503F and the date of service.
- Including the CPT II with the Delivery claim allows for detailed reporting of both the delivery and complexity of services in the postpartum period.
Note: Because the Bundled Obstetric Payment includes Delivery and Postpartum services, CPT 59430 Postpartum Care is not reimbursable for members whose delivery was reported using Bundled Obstetric CPT Codes.
Buckeye Health Plan is excited to announce our new quarterly provider publication Waiver Provider News! This hot of the press communication aims to present news, updates and specialty content that is unique and impactful to our Home and Community Based Service (HCBS) provider network. As those servicing some of our most vulnerable Ohioan’s, Buckeye is committed to engaging in meaningful and impactful ways to strengthen our partnership in service to these individuals. We look forward to growing this through your valuable review and input to ensure this tool provides you value as a resource. Look for an outline of what to expect in our May 2024 Buckeye Provider Bulletin, followed by the first edition launching in our June 2024 Bulletin for Q2.
March 2024
Background
Advancements in the science of genetics and genomics have led to remarkable new options for medical professionals to diagnose, treat, and prevent disease. As genetic testing has increasingly become the standard of care, our health plan is committed to providing the highest levels of access, quality, and value for members in this exciting and dynamic segment of health care.
To achieve these goals, our health plan is asking for your support in the next phase of its genetic testing program. The goals of this phase are twofold -- advance the reliability of laboratory quality information and reduce variability in billing.
Beginning June 1, 2024, Buckeye will expand the requirements for billing of genetic and molecular testing. In accordance with the Reimbursement Policy for Genetic/Molecular Test Coding Policy, all providers billing for genetic and molecular testing services will be required to adhere to the coding recommendation in the Concert Genetics portal. The policies will be posted on our Buckeye Health Plan website for your review by May 1, 2024
The portal can be accessed at Concert Genetics.com/join-centene/. The quality and billing integrity requirements in the reimbursement policy will be facilitated by Concert Genetics--our partner and a software and managed services company that promotes health by providing the digital infrastructure for reliable and efficient management of genetic testing and precision medicine.
What does this mean for our laboratory partners?
We are asking you, our laboratory partner, to do the following:
- Register with Concert Genetics.
- Self-report on quality metrics in a common framework supplied by Concert.
- Verify accuracy of test catalog and view coding recommendations and fee schedule.
- Utilize Concert’s recommended codes when billing for genetic and molecular tests.
Thank you for your support and continued partnership on providing our members with access to high-quality health care at an affordable price.
Effective May 1, 2024, Buckeye Health Plan will be adding prior authorization requirements for the following code:
Service Code | Service/Procedure Description | Line of Business |
G0156 | Services of home health/hospice aide in home health or hospice settings, each 15 minutes. | Medicaid, MMP Duals (for Medicaid reimbursed services) |
Please remember that Buckeye requires ordering, referring, and prescribing provider information on claims as required by the Ohio Department of Medicaid. You may experience claim denials if you are not following the detailed ODM Requirements (PDF)
Buckeye is not currently denying ordering and referring providers when they are not enrolled with the ODM, but we are expected to be doing so in the near future.
Dear Providers,
The Department of Health and Human Services (HHS) will hold an informational session for providers to share how it is responding to the cyberattack on Change Healthcare on Tuesday, March 19, 2024, at 4:00 pm ET. Deputy Secretary Andrea Palm, along with leadership from the Immediate Office of the Secretary and the Centers for Medicare & Medicaid Services (CMS), will lead the session. United Health Group will also attend and discuss their funding program.
You can register for the briefing using this link.
We expect that CMS will provide an overview of how to apply for Medicare advance and accelerated payments, and leaders from UnitedHealth Group will demonstrate how to apply for funding the company has made available to providers. No additional details on the agenda are currently available, but updates will be provided if new information is released.
As a reminder, our Change Healthcare Outage Overview Resource page on Centene.com includes resources and support to help you navigate through this evolving situation, as well as details our approach to provider advances for those facing financial hardship. This page is updated routinely to ensure it is as current and helpful a resource as possible.
Thank you for your continued partnership as we navigate the Change Healthcare outage. If you have any questions, please contact your Provider Engagement representative, visit Centene’s Change Healthcare website or Provider Services: Medicaid 866.296.8731, Wellcare by Allwell 855.766.1851 and Ambetter 877.687.1189.
|
February 2024
Buckeye has updated our member benefits and provider resources for caring for patients with one or more chronic conditions such as asthma, diabetes, hypertension, cardiovascular disease, and sickle cell anemia.
- Transportation Benefit. Buckeye continues to offer no-cost transportation to help members get to medical, dental and vision appointments, as well as to the pharmacy, grocery store, WIC appointments, or the social security administration office.
- Pulmonary Rehabilitation Therapy. Pulmonary rehabilitation offers patients individualized treatment plans that include physical exercises, breathing techniques, nutrition education and counseling.
- Smoking Cessation. Members who are current smokers, or who have been active smokers in the previous 12 months, could benefit from smoking cessation programming. Patients who are ready to quit or are interested in smoking less can contact their Buckeye Care Manager or call Member Services at 866-246-4358.
- Continuous Glucose Monitors (CGM). Where clinically appropriate, Buckeye encourages the use of CGMs to manage diabetes. The Prior Authorization requirement has been lifted through both the Pharmacy (Gainwell) and Durable Medical Equipment benefits to reduce the administrative burden on our providers.
- Diabetes Self-Management Education (DSME). To better support our providers, Buckeye has increased the reimbursement rate paid for the applicable DSME codes (G0108, G0109). Medicaid members who complete DSME may also be eligible for free groceries through our vendor partner Good Measures. Interested patients can be referred to Buckeye Member Services at 866-246-4358.
- Blood Pressure Cuffs – for Home. Medicaid and MMP members with hypertension may receive a blood pressure monitor for their personal use, at no cost to the patient. Interested patients can contact their Buckeye Care Manager or call Member Services at 866-246-4358.
- Sickle Cell Anemia. Does your patient have a sickle cell passport? This includes all the basic information needed for treating Buckeye members diagnosed with sickle cell anemia. Members without this passport should contact their Buckeye Care Manager or call Member Services at 866-246-4358.
Remember, Buckeye provides members with incentives to get good healthcare. They can earn My Health Pays® rewards after completing healthy activities like a yearly wellness exam, annual screenings, exams for children, and vaccinations.
Per CMS Guidelines, effective April 1, 2024, Buckeye Health Plan Medicaid and MyCare plans will begin to deny previously excluded pregnancy diagnosis codes for Coordination of Benefits (COB).
A new federal law was enacted November 14, 2019, to modify Medicaid TPL/Third Party Liability rules related to this special treatment of certain types of care and payment allowing health plans to account for COB. See Federal Policy Guidance.
On February 21, Change Healthcare, a software and data analytics subsidiary of UnitedHealth Group’s Optum unit, experienced a cybersecurity incident that has impacted its network and operations. The cybersecurity incident has created a service disruption impacting our members and provider network in several ways.
As of now, Change Healthcare has not provided a timeline for resolution. To protect our members and providers, we have fully disconnected system access to and from Change Healthcare on February 21, 2024. We are working on multiple solutions to restore provider functionality and ensure continuity of care for our members. We will continue to provide updates as this situation evolves. You can find full details on our website.
Electronic Claim Submission
The ability to electronically submit claims to us through Change Healthcare is currently down. Providers can easily submit electronic claims to us via many alternative methods including other claims clearinghouses, our secure provider portal, and mail. Our preferred clearinghouse for electronic claims submission is Availity. To enroll, please visit the Availity Lifeline page and/or call Availity Client Services at 800-AVAILITY (800-282-4548). For step-by-step instructions for Availity, visit the resource page on our parent company Centene’s Change Healthcare website. Additional information on claims submission can be found in our Provider Manual located on our website.
Reimbursement via Paper Check and Virtual Credit Card (VCC)
Some paper check and virtual credit card payment processes have been disrupted due to this incident. We apologize for any inconvenience this causes. Please know we are working quickly to implement a new process to ensure payments are operational as soon as possible. An alternate way to speed up your payment process is to set up an automated clearing house (ACH) for electronic funds transfer (EFT). Our preferred ACH/EFT partner, PaySpan (now part of Zelis), has offered to help expedite the sign-up process for providers by calling 877-331-7154.
Chart Retrieval
Change Healthcare performs retrieval of medical records for several of our programs, such as HEDIS® and Risk Adjustment. You may have received a request via phone or fax from Change Healthcare where you either scheduled appointments or provided medical records. Change Healthcare is unable to honor any fulfillment requests at this time; however, we will be employing two of our existing medical record retrieval vendors, Datavant (formerly Ciox) and Datafied, to satisfy these prior commitments.
These retrievals are required to report on clinical quality measures and diagnosis data to Health and Human Services (HHS), the Centers for Medicare & Medicaid Services (CMS) and the National Committee for Quality Assurance (NCQA). We ask that you please honor the same commitment dates for these vendors, even if you have already committed to providing medical records to Change Healthcare. If you have provided medical records from the time period of February 19, 2024, to current, you may be asked to provide these medical records again.
Thank you for your patience and partnership as we navigate this situation. We apologize for any inconvenience in this matter. If you have any questions, please contact your Provider Engagement representative, visit Centene’s Change Healthcare website or Provider Services: Medicaid - 866.296.8731, Wellcare by Allwell - 855.766.1851 and Ambetter - 877.687.1189.
January 2024
In 2023, Governor DeWine and ODM Director Maureen Corcoran introduced enhancements to reimbursement rates for providers participating in Ohio’s Medicaid programs. These enhancements were signed into law under HB 33 by both the House and Senate and approved by the Centers of Medicare & Medicaid Services (CMS) effective January 1, 2024.
These increases total approximately $579M, or 5%, across most codes. We are pleased to highlight that the Postpartum Care (CPT 59430) and Transportation Services saw significant increases. You will find the complete list of changes to the Billing Fee Schedule and Rates on the ODM website.
In October, we notified you of changes to the Short Stay policy in response to CMS 2024 rules updates. Medicare Advantage plans are now required to adhere to the CMS 2 Midnight Rule when determining hospital inpatient admission level of care.
In response to the CMS 2024 rules updates Buckeye Health Plan has revised the Short Stay Policy with the following exclusions:
- It is the policy of Medicare health plans affiliated with Centene Corporation® that inpatient hospital stays (vs. observation) spanning less than two midnights are medically necessary when meeting any of the following criteria:
- Admission is for a procedure on the current calendar year CMS Inpatient Only List (2023 addendum E, 2024 addendum E);
- The admitting physician expects the patient to require hospital care that crosses two-midnights based on consideration of complex medical factors documented in the medical record. Such requests will be reviewed on a case-by-case basis by a medical director, considering factors such as member/enrollee history and comorbidities, the severity of signs and symptoms, current medical needs, and the risk of an adverse event occurring during the time period for which hospitalization is considered;
- The admitting physician does not expect the patient to require care that crosses two midnights, but determines, based on complex medical factors documented in the medical record that inpatient care is nonetheless necessary. Such requests will be reviewed on a case-by-case basis by a medical director, considering factors such as member/enrollee history and comorbidities, the severity of signs and symptoms, current medical needs, and the risk of an adverse event occurring during the time period for which hospitalization is considered;1
- Admission to an intermediate or intensive care unit level of care is considered medically necessary per a nationally-recognized clinical decision support tool;
- Admission to acute hospital care at home;
- Unexpected death during the admission;
- Departure against medical advice from a medically necessary (per a nationally-recognized clinical decision support tool) inpatient stay;
- Transferred from another facility, with a medically necessary (per a nationally-recognized clinical decision support tool) total length of stay greater than two days;
- Election of hospice care in lieu of continued treatment in hospital.
- It is the policy of Medicare health plans affiliated with Centene Corporation that inpatient hospital stays on day three and beyond are medically necessary when supported by nationally-recognized clinical decision support tools.
The final rule can be found at the American Hospital Association website.
IMPORTANT UPDATE: Terminations to resume effective January 23, 2024, for failure to complete Medicaid Agreement Revalidations in the Provider Network Management module
If you are currently due for a revalidation in the Provider Network Management (PNM) module, it is imperative that you take immediate action to complete and submit your revalidation application to renew your Ohio Medicaid Provider Agreement. Ohio Department of Medicaid (ODM) will begin terminating providers who fail to complete their revalidation prior to their specified deadline, starting January 23, 2024.
ODM resumed provider revalidation notices in June 2023 as part of the federally required unwinding process from the COVID public health emergency. ODM issues a series of notices with the first one delivered 120 days prior to your Medicaid agreement end date. Subsequent reminders are issued at 90 days, 60 days, and a final notice at 30 days. If you receive a revalidation notice, it is imperative that you take action to complete your revalidation on time. All providers are subject to either three- or five-year time-limited provider agreements.
How do you know if you are due for revalidation?
1. Check your mail and email.
Revalidation reminder notices are mailed and emailed to providers who are due for revalidation prior to the end of their Medicaid agreement. The email will be sent from OHPNM@maximus.com to advise you of a revalidation notice in the PNM Correspondence folder. Please check your spam folder for this email.
2. View the Correspondence folder in the PNM module.
Revalidation notices are posted in the PNM module and can be accessed in the Correspondence folder. Please be sure to select the type of correspondence from the drop down (in this case <Enrollment Notices>), and search for the “Revalidation Notices.” Review the Accessing Communications within PNM Quick Reference Guide for step-by-step instructions.
NOTE: If you think you are due for revalidation but have not received notices, please login to the PNM module and verify that the primary contact information is accurate in accordance with your Ohio Medicaid Provider Agreement. All mailers and email notices are directed to the primary contact individual or address identified in the system.
If I am due for revalidation, what action do I need to take?
A “Begin Revalidation” option appears in the PNM Enrollment Action Selections 120 days prior to the Medicaid Agreement end date. This can be found under the “Manage Application”, “Enrollment Actions” option within the provider file. Review the Revalidation/Reenrollment Quick Reference Guide for step-by-step instructions.
For more information
For technical support or assistance, contact Ohio Medicaid’s Integrated Helpdesk (IHD) at 800-686-1516 and follow the prompts for Provider Enrollment (option two, option two) or email IHD@medicaid.ohio.gov. Representatives are available Monday-Friday, 8:00 a.m.-4:30 p.m. Eastern time.
To learn more about the PNM module and Centralized Credentialing, visit the PNM and Centralized Credentialing page on the Next Generation website.
December 2023
To assist Providers with the prioritization of ePRAF submissions, the Ohio Managed Care Plans (MCPs) have created sustainable interventions through the Technical Assistance Package and Quality Enhancer Incentive Program. The Quality Enhancer Incentive Program provides increased payments to eligible Providers who submit the ePRAF.
Please visit our Pregnancy and Prenatal Information page for more information on these interventions, along with the PRAF 2.0 submission and payment guidelines.
Updates to Diabetes Benefits in 2024
Buckeye and the Ohio Managed Care Organizations (MCOs) are working collaboratively to make diabetes management easier for providers and their patients. Diabetes education and support for the use of continuous glucose monitors (CGMs) have proven to be effective in diabetes care management.
To facilitate increased utilization of these enhanced tools, Buckeye and the other MCOs will pay an enhanced rate to providers rendering Diabetes Self-Management Education (DSME) and billing the appropriate codes: G0108 and G0109. In addition, PA is not required for members who receive a covered CGM device through durable medical equipment (DME) providers or through their pharmacy. Providers must use HCPCS codes A4239 and E2103 for CGMs provided through DME.
For additional information regarding these updates, including who to contact at each MCO for questions, see the quick reference guide.
We are excited to share a change for our Provider Update Newsletter for 2024. To better reflect the importance of the content delivered in our monthly communication, the title will now be: Buckeye Provider Bulletin. 
Beginning October, 2023, providers not enrolled for Medicaid and Marketplace Electronic Funds Transfer (EFT) payments started receiving payment via the Virtual Credit Card (VCC) program.
This program will begin for Medicare payments in 2024.
Going forward all payments will be issued either via electronic funds transfer (EFT) such as PaySpan or the Virtual Credit Card (VCC) program from Change Healthcare.
Change Healthcare is a widely used payment option in healthcare that we are making available to our provider network.
VCC PAYMENTS
VCC payments work like any other credit card payment. You will follow the same process as taking a credit card payment from a patient. Here’s how it works:
- You receive a printed Explanation of Payment that includes a 16-digit card number.
- You enter the number and the full amount of the payment into your credit/debit point-of-sale terminal before the expiration date.
- You receive funds in the same timeframe as your other credit card payments.
- There is no need to enroll to receive VCC payments as they are processed under the merchant agreement with your banking partner.
- Note that your merchant/banking partner charges fees for the payment transaction. These fees are in lieu of the check clearing fees you currently pay.
Providers that had not previously signed up for EFT, were automatically signed up for the VCC when using VCC for other health plans.
You may opt out of VCC at any time by calling 888-678-5862 or via the Echo Payments Simplified website.
If you prefer to enroll in EFT rather than VCC, please go to Providersupport@payspanhealth.com to access the enrollment form and instruction or call 877-331-7154.
We value your ongoing partnership and are excited to offer this new payment option to you.
As required by State and Federal governing entities, Buckeye Health Plan is committed to continuously evaluating and improving overall Payment Integrity solutions. We have partnered with Optum who is supporting us in performing prepayment claim reviews. The purpose of this review is to verify the extent and nature of services rendered for the patient’s condition and claims are coded correctly for services billed.
Optum’s edits will be implemented in phases and are not applied as a blanket “rule” for all claims. This will be implemented for all lines of business – Medicaid, Medicare and Marketplace products.
Only a small number of claims that meet the criteria will be chosen for review. Providers may experience a slight increase in written requests for medical record submission prior to payment. These requests will come from Optum and will contain instructions for providing the documentation. Should the requested documents not be returned, the claim(s) may be denied. Providers will have the ability to dispute findings through Optum directly in the event of a disagreement.
Thank you for your continued participation and cooperation in our ongoing efforts to render quality health care to our members. We look forward to partnering with you to provide the highest quality care for your patients/our members.
Buckeye, in support of Ohio Recovery Housing, is helping to spread the word about new state registration requirements for recovery housing. Providers that operate recovery housing should complete the form through OhioMHAS’ website.
Notable pharmacy changes for Medicare plans coming in 2024
- PBM will be moving from CVS to Express Scripts in 2024 for all Medicare plans. Members have been sent new ID cards with new pharmacy billing information.
- Prior Authorizations will be required for GLP-1 agonists in 2024
- The following table shows the most frequently prescribed drugs that will be non-formulary and their preferred alternatives in 2024:
Top Drug Removals With Alternatives
| |
DRUG(S) REMOVED | FORMULARY ALTERNATIVES |
All MAPD Plans | |
Lantus vial; Lantus SoloStar insulin pen | Basaglar KwikPen insulin pen; Toujeo SoloStar insulin pen; Toujeo Max SoloStar insulin pen; Tresiba vial; Tresiba FlexTouch insulin pen |
Levemir vial; Levemir FlexPen insulin pen | Basaglar KwikPen insulin pen; Toujeo SoloStar insulin pen; Toujeo Max SoloStar insulin pen; Tresiba vial; Tresiba FlexTouch insulin pen |
Victoza pen injector | Bydureon Bcise auto-injector; Mounjaro pen injector; Ozempic pen injector; Rybelsus tablet; Trulicity pen injector |
Byetta pen injector | Bydureon Bcise auto-injector; Mounjaro pen injector; Ozempic pen injector; Rybelsus tablet; Trulicity pen injector |
Flovent Diskus inhalation device; Flovent HFA inhaler (Discontinued by manufacturer) | Arnuity Ellipta inhalation device; Pulmicort Flexhaler aerosol powder |
Betoptic-S suspension eye drops | Alphagan P 0.1% eye drops; Brimonidine Tartrate eye drops; Combigan eye drops |
Kevzara pen injector; Kevzara syringe | Enbrel injection; Humira injection; Rinvoq tablet; Xeljanz tablet; Xeljanz XR tablet (diagnosis dependent) |
Ingrezza capsule | Austedo tablet; Tetrabenazine tablet |
Mavyret tablet; Mavyret pellets in packet | No impact for current utilizers; Epclusa tablets; Epclusa pellets in packet; Harvoni tablets; Harvoni pellets in packet |
D-SNP and MMP Only | |
Simbrinza suspension eye drops | Alphagan P 0.1% eye drops; Brimonidine Tartrate eye drops; Combigan eye drops |
Vyzulta eye drops | Alphagan P 0.1% eye drops; Brimonidine Tartrate eye drops; Combigan eye drops |
November 2023
Step Therapy programs are developed by Wellcare's P&T Committee. They encourage the use of therapeutically equivalent, lower-cost medication alternatives (first-line therapy) before “stepping up” to alternatives that are usually less cost-effective.
Step Therapy programs are intended to be a safe and effective method of reducing the cost of treatment by ensuring that an adequate trial of a proven safe and cost-effective therapy is attempted before progressing to a more costly option. First-line drugs are recognized as safe, effective, and economically sound treatments.
The first-line drugs on Wellcare’s formulary have been evaluated through the use of clinical literature and are approved by Wellcare’s P&T Committee. Step therapy is failure of at least one different or less expensive drug prior to coverage of a drug on this list.
Drugs requiring step therapy effective January 01, 2024 are listed below. The prescriber, patient, or authorized representative may ask for an exception. Step therapy applies if the drug has not been used in the past 365 days.
Drug Name
- Abatacept (Orencia®)
- Ado-trastuzumab emtansine (Kadcyla®)
- Aflibercept (Eylea®)
- Atezolizumab (Tecentriq®)
- Axicabtagene ciloleucel (Yescarta®)
- Bevacizumab (Avastin®, Alymsys®, Mvasi®, Vegzelma™, Zirabev™)
- Brentuximab vedotin (Adcetris®)
- Brexucabtagene autoleucel (Tecartus™)
- Brolucizumab-dbll (Beovu®)
- Cemiplimab-rwlc (Libtayo®)
- Certolizumab (Cimzia®)
- Ciltacabtagene autoleucel (Carvykti™)
- Corticosteroid intravitreal implants: dexamethasone (Ozurdex®), fluocinolone acetonide (Iluvien®, Retisert®, Yutiq™)
- Corticotropin (H.P. Acthar®, Purified Cortrophin™ Gel)
- Daratumumab (Darzalex®), daratumumab/hyaluronidase-fihj (Darzalex Faspro™)
- Darbepoetin alfa (Aranesp®)
- Denosumab (Xgeva®)
- Durvalumab (Imfinzi®)
- Eflapegrastim-xnst (Rolvedon™)
- Elotuzumab (Empliciti®)
- Emapalumab-lzsg (Gamifant™)
- Epoetin alfa (Epogen®, Procrit®)
- Faricimab-svoa (Vabysmo™)
- Ferric carboxymaltose (Injectafer®)
- Ferric derisomaltose (Monoferric®)
- Ferric pyrophosphate (Triferic®, Triferic Avnu®)
- Ferumoxytol (Feraheme®)
- Filgrastim (Neupogen®, Zarxio®, Nivestym™, Granix®, Releuko®)
- Golimumab (Simponi®, Simponi Aria®)
- Hyaluronate derivatives: sodium hyaluronate (Euflexxa®, Gelsyn-3™, GenVisc®850, Hyalgan®, Supartz FX™, Synojoynt™, Triluron™, TriVisc™, VISCO-3™), hyaluronic acid (Durolane®), cross-linked hyaluronate (Gel-One®), hyaluronan (Hymovis®, Orthovisc®, Monovisc®), hylan polymers A and B (Synvisc®, Synvisc One®)
- Idecabtagene vicleucel (Abecma™)
- Immune globulins (Asceniv™, Bivigam®, Cutaquig®, Cuvitru™, Flebogamma® DIF, GamaSTAN®, GamaSTAN® S/D, Gammagard® liquid, Gammagard® S/D, Gammaked™, Gammaplex®, Gamunex®-C, Hizentra®, HyQvia®, Octagam®, Panzyga®, Privigen®, Xembify®)
- IncobotulinumtoxinA (Xeomin®)
- Lisocabtagene maraleucel (Breyanzi®)
- Lurbinectedin (Zepzelca™)
- Luspatercept-aamt (Reblozyl®)
- Lutetium Lu 177 dotatate (Lutathera®)
- Nadofaragene firadenovec-vncg (Adstiladrin®)
- Natalizumab (Tysabri®)
- Nivolumab (Opdivo®)
- Pegfilgrastim (Neulasta®, Fulphila™, Fylnetra®, Nyvepria™, Stimufend®, Udenyca™, Ziextenzo™)
- Pembrolizumab (Keytruda®)
- Polatuzumab vedotin-piiq (Polivy™)
- Ramucirumab (Cyramza®)
- Ranibizumab (Lucentis®, Byooviz®, Cimerli™, Susvimo™)
- RimabotulinumtoxinB (Myobloc®)
- Rituximab (Rituxan®, Riabni™, Ruxience™, Truxima®), rituximab/hyaluronidase (Rituxan Hycela™)
- Romiplostim (Nplate®)
- Romosuzumab-aqqg (Evenity™)
- Sargramostim (Leukine®)
- Sipuleucel-T (Provenge®)
- Teclistamab-cqyv (Tecvayli®)
- Teprotumumab-trbw (Tepezza™)
- Tisagenlecleucel (Kymriah®)
- Tocilizumab (Actemra®)
- Trastuzumab (Herceptin®, Ontruzant®, Herzuma®, Ogivri™, Trazimera™, Kanjinti™), trastuzumab/hyaluronidase (Herceptin Hylecta™)
- Triamcinolone ER injection (Zilretta®)
- Triamcinolone acetonide suprachoroidal injection (Xipere™)
- Vedolizumab (Entyvio®)
- Verteporfin (Visudyne®)
We are pleased to announce that, effective January 1, 2024, Express Scripts® will begin processing pharmacy claims for our plan members.
Express Scripts is a pharmacy benefit management (PBM) company serving more than 100 million Americans. Express Scripts Pharmacy delivers specialized care that puts patients first through a smarter approach to pharmacy services.
Members have been notified in advance and will receive a new ID card with updated pharmacy information, so that they are prepared to begin having their prescriptions filled at participating network pharmacies when this change occurs.
Providers can direct members to call the Member Services phone number listed on their ID card should they have questions about this change.
Please contact your Provider Engagement Administrator with any additional questions.
Thank you for the care you provide to our members.
Buckeye Health Plan is pleased to share that we will be transitioning from Optum to 6 Degrees Health for our clean claim reviews. The Go-Live launch is tentatively set for January 2, 2024.
This transition will be seamless for our provider community and reflects our ongoing efforts to make it easier to do business with Buckeye. 6 Degrees offers providers the ability to submit records via mail, fax or email.
You may begin seeing record request correspondence from 6 Degrees Heath.
ODM has asked us to remind you that Provider Network Management (PNM) is continuing our provider awareness and training efforts. Registration for the November 6-16 PNM module refresher training is now open. The training schedule is available on the PNM and Centralized Credentialing page. Below you will find how to register and a list of training topics.
New Sandata Mobile Connect (SMC) application for EVV Providers
A new Sandata Mobile Connect (SMC) application was released in application (app) stores in July 2023. This is an enhanced app with a focus on the end user experience. In addition to an updated look and feel that simplifies navigation, the update includes a simplified login process and efficient reset password process. For providers using their own devices, the old app will be available until June 30, 2024. Between now and then, no updates will be available for the old app, so be sure to download the new one.
The new SMC app can now be downloaded in either Android or Apple stores:
Information on the new SMC app is available online at Sandata on Demand.
Reminder: Nursing Facility Claims Billing Value Code 31
For Nursing facility claims, Value code 31 should only be used to indicate a Lump Sum amount, and not the individual’s monthly patient liability amount as indicated in OAC 5160-3-39.1. Buckeye Health Plan will separately apply any amount billed under value code 31, on nursing claims as a separate Lump sum payment. The Lump Sum amount will be applied in addition to the member’s monthly liability indicated to Buckeye by ODM.
Value code 31 should not be used to indicate the member’s monthly liability. For additional guidance on billing the appropriate member liability codes, please see Ohio Medicaid companion Guides
October 2023
Effective January 1, 2024, Buckeye Health Plan is expanding our prior authorization program to include non-emergent MSK procedures. The expansion includes inpatient and outpatient hip, knee, shoulder, lumbar and cervical spine surgeries for Buckeye Health Plan members.
We are pleased to announce a partnership with National Imaging Associates, Inc (NIA)* for utilization management services for non-emergent, Medical Specialty Solutions. In consideration of the aforementioned agreement, Buckeye Health Plan will terminate its current MSK program and utilization management efforts with TurningPoint as of December 31, 2023.
Under the terms of the agreement between Buckeye Health Plan and NIA, Buckeye Health Plan will oversee the MSK program and continue to be responsible for claims adjudication and medical policies. NIA will manage non-emergent outpatient interventional spine pain management services, and inpatient and outpatient MSK surgeries through the existing contractual relationships with Buckeye Health Plan.
Planned for a January 1, 2024, implementation, this announcement serves as notice under your participating Buckeye Health Plan Provider Agreement of changes to the program.
Providers may begin contacting NIA on January 1, 2024, to seek prior authorization for procedures scheduled on or after January 1, 2024.
The following outlines the specific procedures requiring prior authorization.
MSK Surgeries
Prior authorization will be required for the following non-emergent inpatient and outpatient hip, knee, shoulder, lumbar and cervical surgeries:
Hip
- Revision/Conversion Hip Arthroplasty
- Total Hip Arthroplasty/Resurfacing
- Femoroacetabular Impingement (FAI) Hip Surgery (includes CAM/pincer & labral repair)
- Hip Surgery – Other (includes synovectomy, loose body removal, debridement, diagnostic hip arthroscopy, and extra-articular arthroscopy knee)
Knee
- Revision Knee Arthroplasty
- Total Knee Arthroplasty (TKA)
- Partial-Unicompartmental Knee Arthroplasty (UKA)
- Knee Manipulation under Anesthesia (MUA)
- Knee Ligament Reconstruction/Repair
- Knee Meniscectomy/Meniscal Repair/Meniscal Transplant
- Knee Surgery – Other (includes synovectomy, loose body removal, diagnostic knee arthroscopy, debridement with or without chondroplasty, lateral release/patellar realignment, articular cartilage restoration)
Shoulder
- Revision Shoulder Arthroplasty
- Total/Reverse Shoulder Arthroplasty or Resurfacing
- Partial Shoulder Arthroplasty/Hemiarthroplasty
- Shoulder Rotator Cuff Repair
- Shoulder Labral Repair
- Frozen Shoulder Repair/Adhesive Capsulitis
- Shoulder Surgery – Other (includes debridement, manipulation, decompression, tenotomy, tenodesis, synovectomy, claviculectomy, diagnostic shoulder arthroscopy)
Lumbar
- Lumbar Microdiscectomy
- Lumbar Decompression (Laminotomy, Laminectomy, Facetectomy & Foraminotomy)
- Lumbar Spine Fusion (Arthrodesis) With or Without Decompression – Single & Multiple Levels
- Lumbar Artificial Disc Replacement
- Sacroiliac Joint Fusion
Cervical
- Cervical Anterior Decompression with Fusion –Single & Multiple Levels
- Cervical Posterior Decompression with Fusion –Single & Multiple Levels
- Cervical Posterior Decompression (without fusion)
- Cervical Artificial Disc Replacement
- Cervical Anterior Decompression (without fusion)
KEY PROVISIONS:
- It is the responsibility of the ordering physician to obtain prior authorization for all interventional spine pain management procedures and MSK surgeries outlined above.
- NIA does not manage prior authorization for emergency MSK surgery cases that are admitted through the emergency room or for MSK surgery procedures outside of those procedures listed above.
- Any Buckeye Health Plan prior authorization requirements for the facility or hospital admission must be obtained separately and only initiated after the surgery/procedure has met NIA’s medical necessity criteria.
Services other than MSK surgeries outlined above will continue to follow Buckeye Health Plan prior-authorization requirements for hospital admissions and elective surgeries.
We appreciate your support and look forward to your assistance in assuring that Buckeye Health Plan members receive MSK services delivered in a quality, clinically appropriate fashion.
We will provide additional information as we get closer to the implementation date. Should you have questions at this time, please contact Buckeye Health Plan Provider Services Department at 1-866-246-4359.
* Effective 1/20/2023, National Imaging Associates, Inc. is now a subsidiary of Evolent Health. Evolent Health and its affiliates and subsidiaries collectively referred to as “Evolent.”
New Policies for Medicare
• MC.CP.MP.170 Peripheral Nerve Blocks
• MC.CP.MP.22 Stereotactic Body Radiation Therapy
• MC.CP.MP.69 Intensity-Modulated Radiotherapy
• MC.CP.MP.246 Pediatric Kidney Transplantation
• MC.CP.MP.57 Lung Transplantation
• MC.CP.MP.101 Donor Lymphocyte Infusion
• MC.CP.MP.108 Allogeneic Hematopoietic Cell Transplants for Sickle Cell Anemia and Beta-thalassemia
• MC.CP.MP.182 Short Inpatient Stay
• MC.CP.MP.106 Endometrial Ablation
• MC.CP.MP.160 Wireless Pulmonary Artery Monitoring
• CP.PP.206 Skilled Nursing Facility Leveling
• V2.2023 Concert Genetics Genetic Testing Aortopathies and Connective Tissue Disorders
• V2.2023 Concert Genetics Genetic Testing Cardiac Disorders
• V2.2023 Concert Genetics Genetic Testing Dermatologic Conditions
• V2.2023 Concert Genetics Genetic Testing Epilepsy, Neurodegenerative, and Neuromuscular Conditions
• V2.2023 Concert Genetics Genetic Testing Exome and Genome Sequencing for the Diagnosis of Genetic Disorders
• V2.2023 Concert Genetics Genetic Testing Eye Disorders
• V2.2023 Concert Genetics Genetic Testing Gastroenterologic Disorders (non-cancerous)
• V2.2023 Concert Genetics Genetic Testing General Approach to Genetic Testing
• V2.2023 Concert Genetics Genetic Testing Hearing Loss
• V2.2023 Concert Genetics Genetic Testing Hematologic Condition (non-cancerous)
• V2.2023 Concert Genetics Genetic Testing Hereditary Cancer Susceptibility
• V2.2023 Concert Genetics Genetic Testing Immune, Autoimmune, and Rheumatoid Disorders
• V2.2023 Concert Genetics Genetic Testing Kidney Disorders
• V2.2023 Concert Genetics Genetic Testing Lung Disorders
• V2.2023 Concert Genetics Genetic Testing Metabolic, Endocrine, and Mitochondrial Disorders
• V2.2023 Concert Genetics Genetic Testing Multisystem Inherited Disorders, Intellectual Disability, and Developmental Delay
• V2.2023 Concert Genetics Genetic Testing Non-Invasive Prenatal Screening (NIPS)
• V2.2023 Concert Genetics Genetic Testing Pharmacogenetics
• V2.2023 Concert Genetics Genetic Testing Preimplantation Genetic Testing
• V2.2023 Concert Genetics Genetic Testing Prenatal and Preconception Carrier Screening
• V2.2023 Concert Genetics Genetic Testing Prenatal Diagnosis via Amniocentesis, CVS or PUBS and Pregnancy Loss
• V2.2023 Concert Genetics Genetic Testing Skeletal Dysplasia and Rare Bone Disorders
• V2.2023 Concert Genetics Oncology Algorithmic Testing
• V2.2023 Concert Genetics Oncology Cancer Screening
• V2.2023 Concert Genetics Oncology Circulating Tumor DNA and Circulating Tumor Cells Liquid Biopsy
• V2.2023 Concert Genetics Oncology Cytogenetic Testing
• V2.2023 Concert Genetics Oncology Molecular Analysis of Solid Tumors and Hematologic Malignancies
Policies with updates
• CP.CPC.05 Medical Necessity Criteria Hierarchy
• Clinical Practice Guidelines
Policies Retired for Medicare
• CP.MP.100 Allergy Testing and Therapy
• CP.MP.101 Donor Lymphocyte Infusion
• CP.MP.102 Pancreas Transplant
• CP.MP.105 Digital EEG Analysis
• CP.MP.106 Endometrial Ablation
• CP.MP.107 Durable Medical Equipment and Orthotics and Prosthetics Guidelines
• CP.MP.108 Allogeneic Hematopoietic Cell Transplants For Sickle Cell Anemia and Beta-thalassemia
• CP.MP.109 Panniculectomy
• CP.MP.110 Bronchial Thermoplasty
• CP.MP.113 Holter Monitors
• CP.MP.114 Disc Decompression Procedures
• CP.MP.115 Discography
• CP.MP.116 Lysis of Epidural Lesions
• CP.MP.117 Spinal Cord Stimulation
• CP.MP.12 Vagus Nerve Stimulation
• CP.MP.120 Pediatric Liver Transplant
• CP.MP.121 Homocysteine Testing
• CP.MP.123 Laser Therapy for Skin Conditions
• CP.MP.126 Sacroiliac Joint Infusion
• CP.MP.127 Total Artificial Heart
• CP.MP.128 Optic Nerve Decompression Surgery
• CP.MP.129 Fetal Surgery in Utero for Prenatally Diagnosed Malfunctions
• CP.MP.130 Fertility Preservation
• CP.MP.132 Heart-Lung Transplant
• CP.MP.133 Posterior Tibial Nerve Stimulation for Voiding Dysfunction
• CP.MP.134 Evoked Potential Testing
• CP.MP.136 Home Births
• CP.MP.137 Fecal Incontinence Treatments
• CP.MP.138 Pediatric Heart Transplant
• CP.MP.139 Low-frequency Ultrasound and Noncontact Wound Therapy
• CP.MP.14 Cochlear Implant Replacements
• CP.MP.141 Non-myeloablative Allogeneic Stem Cell Transplants
• CP.MP.142 Urinary Incontinence Devices and Treatments
• CP.MP.143 Wireless Motility Capsule
• CP.MP.144 Mechanical Stretching Devices for Joint Stiffness and Contracture
• CP.MP.145 Electric Tumor Treating Fields (Optune)
• CP.MP.146 Sclerotherapy for Chemical Endovenous Ablation for Varicose Veins
• CP.MP.147 Percutaneous Left Atrial Appendage Closure Device for Stroke Prevention
• CP.MP.148 Radial Head Implant
• CP.MP.150 Phototherapy for Neonatal Hyperbilirubinemia
• CP.MP.151 Transcatheter Closure of Patent Foramen Ovale
• CP.MP.152 Measurement of Serum 1,25-dihydroxyvitamin D
• CP.MP.153 Helicobacter Pylori (H Pylori) Serology Testing
• CP.MP.154 Thyroid Hormones and Insulin in Pediatrics
• CP.MP.155 EEG Headache
• CP.MP.156 Cardiac Biomarker Testing
• CP.MP.157 25-hydroxyvitamin D Testing in Children and Adolescents
• CP.MP.158 Ambulatory Surgery Center Optimization
• CP.MP.160 Implantable Wireless Pulmonary Artery Pressure Monitoring
• CP.MP.162 Tandem Transplant
• CP.MP.163 Total Parenteral Nutrition and Intradialytic Parenteral Nutrition
• CP.MP.164 Caudal or Interlaminar Epidural Steroid Injections
• CP.MP.165 Selective Nerve Root Blocks and Transforaminal Epidural Injections
• CP.MP.166 Sacroiliac Joint Interventions for Pain Management
• CP.MP.167 Intradiscal Steroid Injections for Pain Management
• CP.MP.168 Biofeedback
• CP.MP.169 Trigger Point Injections for Pain Management
• CP.MP.170 Nerve Blocks for Pain
• CP.MP.171 Facet Joint Interventions
• CP.MP.173 Implantable Intrathecal or Pain Pump
• CP.MP.174 Selective Dorsal Rhizotomy for Spasticity in Cerebral Palsy
• CP.MP.175 Air Ambulance
• CP.MP.176 Outpatient Cardiac Rehabilitation
• CP.MP.180 Implantable Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea
• CP.MP.181 Polymerase Chain Reaction Respiratory Viral Panel Testing
• CP.MP.182 Short Inpatient Hospital Stay
• CP.MP.184 Home Ventilators
• CP.MP.185 Skin and Soft Tissue Substitutes for Chronic Wounds
• CP.MP.186 Burn Surgery
• CP.MP.188 Pediatric Oral Function Therapy
• CP.MP.190 Outpatient Oxygen Use
• CP.MP.194 Osteogenic Stimulation
• CP.MP.202 Orthognathic Surgery
• CP.MP.203 Diaphragmatic Phrenic Nerve Stimulation
• CP.MP.206 Skilled Nursing Facility Leveling
• CP.MP.209 Gastrointestinal Pathogen Nucleic Acid Detection Panel Testing
• CP.MP.210 Repair of Nasal Valve Compromise
• CP.MP.22 Stereotactic Body Radiation Therapy
• CP.MP.24 Multiple Sleep Latency Testing
• CP.MP.242 Pulmonary Function Testing
• CP.MP.243 Implantable Loop Recorder
• CP.MP.244 Liposuction for Lipedema
• CP.MP.246 Pediatric Kidney Transplant
• CP.MP.247 Transplant Service Documentation Requirements
• CP.MP.248 Facility Based Sleep Studies for OSA
• CP.MP.26 Articular Cartilage Defect Repair
• CP.MP.31 Cosmetic and Reconstructive Surgery
• CP.MP.36 Experimental Technologies
• CP.MP.37 Bariatric Surgery
• CP.MP.38 Ultrasound in Pregnancy
• CP.MP.40 Gastric Electrical Stimulation
• CP.MP.43 Functional MRI
• CP.MP.46 Ventricular Assist Devices
• CP.MP.48 Neuromuscular and Peroneal Nerve Electric Stimulation
• CP.MP.49 Physical Occupational and Speech Therapy Services
• CP.MP.50 Drugs of Abuse Definitive Testing
• CP.MP.51 Reduction Mammoplasty and Gynecomastia Surgery
• CP.MP.53 Ferriscan R2-MRI
• CP.MP.54 Hospice
• CP.MP.55 Assisted Reproductive Technology
• CP.MP.57 Lung Transplantation
• CP.MP.58 Intestinal and Multivisceral Transplant
• CP.MP.61 IV Moderate Sedation IVE Deep Sedation and General Anesthesia for Dental Procedures
• CP.MP.62 Hyperhidrosis Treatments
• CP.MP.69 Intensity Modulated Radiotherapy
• CP.MP.70 Proton and Neutron Beam Therapies
• CP.MP.71 Long Term Care Placement Criteria
• CP.MP.81 NICU Discharge Guidelines
• CP.MP.82 NICU Apnea Bradycardia
• CP.MP.85 Neonatal Sepsis Management
• CP.MP.86 Neonatal Abstinence Syndrome Guidelines
• CP.MP.87 Therapeutic Utilization of Inhaled Nitric Oxide
• CP.MP.91 Obstetrical Home Care Programs
• CP.MP.92 Acupuncture
• CP.MP.93 Bone-Anchored Hearing Aid
• CP.MP.94 Clinical Trials
• CP.MP.95 Gender-Affirming Procedures
• CP.MP.98 Urodynamic Testing
• CP.MP.99 Wheelchair Seating
• CP.BH.100 Substance Use Disorders and Treatment Services
• CP.BH.104 Applied Behavioral Analysis
• CP.BH.124 ADHD
• CP.BH.200 TMS for Treatment Resistant Major Depression
• CP.BH.201 Deep Transcranial Magnetic Stimulation for Treatment of Obsessive-Compulsive Disorder
• CP.BH.300 Biofeedback for BH Disorders
We are writing to address a critical issue affecting some of our accounts within the provider portal and to provide guidance on how to resolve it promptly.
Buckeye Password Policy
As part of our commitment to maintaining the highest level of security for our government partners, the provider portal enforces a 'One Year Password Policy.' This policy mandates that passwords be changed before 365 days. Failure to update passwords within the specified timeframe results in the user’s account locking.
Buckeye is proactively working to identify ‘locked’ accounts and unlock them. If you have NOT reset your password within the designated timeframe, you may now be locked out.
Resolution and Next Steps
Step 1: Please go to the Buckeye Provider Portal and attempt to login:
- If you are NOT locked out, you need to take immediate action to reset your password.
- If you ARE locked out, you need to follow these steps.
Step 2: If you are NOT locked out:
To prevent any lock out, please do the following on the Portal Login page:
- Click on 'Trouble Logging In' on the portal login page.
- Follow the "Forgot Password" process to reset your password.
Step 2: If you ARE locked out:
If your account is locked, you will see the Account Recovery screen.
In addition, you will receive an error message and an email that notifies you that your password change was NOT successful. Please take the following steps:
- Reach out to the call center at 866.296.8731: The call center will raise an incident ticket which will help us unlock your account.
- Reset Password: To regain access to the account, users must reset their password. Here's how to reset:
- On the Portal Login page, click on 'Trouble Logging In'.
- Follow the "Forgot Password" process to reset password.
- Important: After resetting your password, your account will be reactivated.
If you have any problems, please contact Provider Services at: 866.296.8731.
Buckeye is in receipt of the below notice from ODM. We understand many of you have already seen and your Clinical Engineering departments have acted on this notice, but Buckeye wants to ensure you are aware:
ODM would like to make the MCEs aware of the below critical recall from the FDA.
The full announcement is available on the FDA's website.
Hamilton Medical Inc. Recalls HAMILTON-C1, T1, MR-1 Ventilators for Capacitator Leaks and Short Circuits
Hamilton Medical, Inc. is recalling the HAMILTON-C1, T1, MR-1 ventilators because the capacitators may leak electrolyte fluid onto the ventilator’s control board. If the control board contacts the electrolyte fluid, the control board or installed spare parts could short circuit. As a result of the short circuit, the ventilator may switch to “Ambient State.”
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Questions?
If you have questions about this recall, contact Hamilton Medical Inc. at 1-800-426-6331 or email reno.techsupport@hamiltonmedical.com.
When providers follow appointment access standards, emergency room visits decrease, health outcomes improve and patient trust in their primary care provider (PCP) increases.
- What are appointment access standards?
These standards ensure members have timely access to care, so they get the right care, at the right time, in the right location.
- Why do appointment access standards matter?
Providers are required to follow the guidelines that are outlined by government agencies and in their participating provider agreement. This helps to reduce unnecessary emergency room visits and increases patient engagement with their PCP.
- How can Health Plans help providers meet the appointment access standards?
Health Plan staff should have a full understanding of the standards and encourage providers to follow these access standards. Providers should adhere to these required timeframes when they schedule appointments.
Refer to your Provider Manual for additional information pertaining to appointment access standards.

Centene CEO Sarah London was selected for Fortune's 2023 "Most Powerful Women," coming in at No. 40 on the list.
Sarah joins 100 women leaders, including 67 women CEOs, from organizations such as CVS, General Motors and UPS. The ranking includes the following criteria — the size and importance of the leader's business in the global economy, the health and direction of the business, the arc of the woman's career, her social and cultural influence, and how she shapes her company and the world.
Since becoming Centene's CEO in 2022, Sarah has been instrumental in guiding the organization to realize its vision to transform the health of the communities we serve. She has focused the organization on its three core lines of business and invested in programs and technology that support our ability to improve health and access to high-quality care for the members and communities we serve.
This year, Sarah was also named one of Modern Healthcare's Top Women Leaders in Healthcare 2023. The program honors female executives who are leading change, developing policy and guiding healthcare delivery improvement. The distinction recognizes leaders from all sectors of the industry for their professional accomplishments and contributions to their organizations.
The youngest female CEO on the Fortune 500, Sarah also took part in a Fortune interview and videos where she discussed leadership, teamwork and culture.
The Comprehensive Maternal Care (CMC) re-attest links in PNM are currently not working correctly
ODM's vendor for the Provider Network Management (PNM) module is already working on this known issue. This system bug is impacting the Medicaid provider file for those that are currently participating in CMC for the 2023 program year. If a participating Medicaid ID received an invitation for next year, we recommend ensuring that users have the proper access needed and are able to see the CMC re-attest link, but to refrain from clicking on the link until the system has been fixed.
At this time, we do not have an ETA for resolution. Additional communication will be sent once more information is known.
This is not impacting any Medicaid ID that would be enrolling in CMC for the first time. Those enrollment actions are available and working as expected. We highly recommend completing those enrollments while awaiting the system fix for continuing practices.
Ohio Department of Medicaid (ODM) is aware trading partners are submitting Electronic Data Interchange (EDI) transactions with more than 5,000 CLM segments. Submissions of over 5,000 CLM segments are causing downstream issues and affecting the delivery of corresponding 277CAs back to trading partners.
Trading partners should remember to limit their transaction size to less than 5,000 CLM segments as required by ODM Companion Guides. This best practice is outlined in the Technical Reports Type 3 (TR3) which recommends “that trading partners limit the size of the transaction (ST-SE envelope) to a maximum of 5,000 CLM segments.” This limitation also applies to the submission of batch 270 eligibility inquiries and 276 claim status inquiries.
Trading partners who have submitted more than 5,000 CLM segments in an EDI file and have not received a rejection via 999 or 824 AND have not received a corresponding 277CA and 835, should resubmit those claims.
September 2023
Effective October 20, Next Generation Medicaid managed care organizations (MCO), the OhioRISE plan, and MyCare Ohio plans must use provider data from Ohio Medicaid’s Provider Network Management (PNM) module as it is the official system of record. To ensure the provider data sent from the PNM to the managed care entities (MCE) is accurate, it is imperative that your records are updated within the PNM module. If your data in the PNM module does not match your data on the submitted claim, your claims will be denied for payment.
Actions needed by you as a provider or a trading partner
- Check that all data submitted on a claim and within the PNM module (e.g., addresses, affiliations, specialties, locations) is accurate and up to date.
- Access Provider Education & Training Resources within the PNM ‘Learning’ tab for step-by-step instructions.
- Continue to update data as changes occur. MCEs will use this information as the system of record moving forward.
The MCEs download a complete extract of the Provider Master File (PMF) that includes all provider data daily.
For more information
For technical support or assistance, contact Ohio Medicaid’s Integrated Helpdesk (IHD) at 800-686-1516 and follow the prompts for Provider Enrollment (option 2, option 2) or email IHD@medicaid.ohio.gov. Representatives are available Monday-Friday, 8 a.m.-4:30 p.m. Eastern time.
To learn more about the PNM module and Centralized Credentialing, visit the PNM and Centralized Credentialing page on the Next Generation website.
This year, Ohio Department of Medicaid (ODM) will be hosting a virtual CPC Fall Learning Collaborative in place of the Annual Summer Learning Session.
Will be held on Friday, October 20, from 9:30 a.m.-12:30 p.m. via GoToWebinar. If you are interested in attending, register here for webinar.
Some of the topics to be covered include:
- Risk stratification
- Quality and efficiency metrics
- CPC and managed care organization (MCO) collaboration
- IPRO activity monitoring reviews
There will also be break out group discussions on CPC operationalization with your peers, from similar facility sizes and types.
ODJFS Rolls Out E-Signature Feature for Ohio Benefits Recipients
The Ohio Department of Job and Family Services (ODJFS) Director Matt Damschroder announced on September 13, 2023, a new electronic signature process that will save Ohioans time when applying for certain benefits by phone.
“Beginning today, residents in all 88 counties will be able to apply, renew, or recertify for Medicaid, SNAP, cash assistance, and publicly funded childcare right from their smart phone,” said Damschroder. “The e-signature program is not mandatory, but it will be a time-saver for those who participate.”
The new e-signature process allows customers to receive a link on their smart phone, review their Rights and Responsibilities and sign in real time, which is instantly transferred to Ohio Benefits, the system used to manage food, cash, medical, and childcare assistance. It is an alternative to the existing telephonic signature process and could save up to 20 minutes for the applicant on the phone.
Summit, Ross, Vinton, and Hocking counties have been piloting e-signatures since early July. Cuyahoga and Franklin counties started in early August and Columbiana, Coshocton, Fairfield, Guernsey, Licking, Monroe, Muskingum, Noble, Perry, and Wayne counties rolled out the program August 31st.
“We’ve had a great deal of positive feedback from both counties and customers during the pilot period,” said Damschroder. “We are pleased to make this available as part of our ongoing efforts to improve the customer service for those Ohioans we serve.”
Customers can still mail, drop off, or fax applications to their county office to apply, renew, or recertify their benefits, or they can complete the same applications through the online Self-Service Portal (SSP).
Modifier SA is used when the Nurse Practitioner (NP) is assisting with any other procedure that does not include surgery in accordance with MTL No. 3336-18-01. This is currently a requirement for Medical claims, and in order to better align OH Medical with Behavioral health services, Buckeye will now require the SA modifier for non-84/95 provider types on the Behavioral health services beginning 11/01/2023. Provider claims where the Nurse Practitioner is assisting with any other procedure that does not include surgery that are not billed with the SA modifier will be denied beginning 11/01/2023.
All Ohio Department of Medicaid (ODM) providers are required to revalidate or renew your ODM provider agreement every three years or five years. Credentialed provider types are subject to three-year provider agreements and are both revalidated and recredentialed at that interval. All other providers are subject to five-year provider agreements and must revalidate before the end date of that agreement to continue participation in Ohio Medicaid as an active enrolled provider.
ODM mails and emails a reminder notice to the contact listed on the Primary Contact page in the Provider Network Management (PNM) module 120 days before the Ohio Medicaid provider agreement expires. To ensure you receive these notices, you must maintain a current mailing and email address.
Review the “2023 Upcoming Revalidations” file
To provide additional support and information about provider revalidation schedules, ODM has published a 2023 Upcoming Revalidations file on the Ohio Medicaid website and can be found here. This report provides a complete list of all revalidations due in 2023. The revalidation list contains the provider’s name and National Provider Number (NPI) or Medicaid ID.
Once the revalidation workflow in PNM is initiated within the 120-day provider agreement revalidation timeframe, providers cannot initiate any other workflows to perform PNM updates (i.e., affiliations, demographic updates, requesting a new specialty, etc.) until the revalidation is complete and approved. However, updates or changes may still be made within the revalidation workflow as part of the revalidation verification process.
Note: ODM will publish a revalidation list for 2024 in early October 2023.
For More Information
For technical support or assistance, contact ODM’s Integrated Helpdesk (IHD) at 800-686-1516 and follow the prompts for Provider Enrollment (option 2, option 2) or email IHD@medicaid.ohio.gov. Representatives are available 8 a.m.-4:30 p.m. Eastern time Monday-Friday.
To learn more about the PNM module and Centralized Credentialing, visit the PNM and Centralized Credentialing page on the Next Generation website.
August 2023
Buckeye Grievances & Appeals is looking to continue the trend of making Buckeye easier to do business with. Following Prior Authorization policies will minimize the chances of needing an Appeal. Please review the key steps on our Prior Authorization website page.
Help Your Medicaid/MyCare Patients get their Incontinence Supplies Faster
When ordering incontinence supplies, remember to indicate both a primary diagnosis and the type of incontinence on the prescription. This is an ODM requirement. Without both items, the order cannot be completed and your patients will not receive their supplies.
Effective October 1st, 2023, there are changes to prior authorization requirements. Please see the PDF document below.
For complete CPT/HCPCS code listing, please see Online Prior Authorization Tool.
Babylon Health’s telehealth services are no longer available as of August 7, 2023. Due to this change, you may receive new requests for both virtual and in-person services from Ambetter Virtual Access members as you are currently in our network.
As always, please verify each member's eligibility, benefits, and referral requirements before rendering care. You can find an example of an Ambetter Virtual Access member ID card on our website.
Please contact Provider Services at 877-687-1189 with any questions.
In an effort to eliminate administrative burden for facility-based Hospice services, Buckeye Health Plan is removing prior authorization requirements for the following service codes, effective September 30, 2023.
NON-PAR PROVIDERS REQUIRE AUTHORIZATION FOR ALL HOSPICE SERVICES EXCEPT WHERE INDICATED
Service Code | Service/Procedure Description | Line of Business |
|---|---|---|
T2044 | Hospice inpatient respite care | Medicaid, MMP Duals |
T2045 | Hospice general inpatient care | Medicaid |
T2046 | Hospice long term care, room and board only | Medicaid, MMP- Duals |
Important information on clinical diagnostic claims
Ohio Department of Medicaid (ODM) is aware that some claims for clinical diagnostic laboratory services are currently not being paid because system edit 103 has determined that the procedure reported on a detail line is "not an approved service for provider".
This problem is occurring because the Fiscal Intermediary (FI) is attempting to verify the Clinical Laboratory Improvement Amendments (CLIA) certification only of the rendering provider (e.g., individual practitioner) and not of the billing provider (e.g., professional medical group).
Claims with detail lines for clinical diagnostic laboratory services that are affected by edit 103 were in 'pending' status but have been released for processing, at which point payment for the pended details will be paid or denied.
ODM is working with our vendor on a solution to this issue. We will provide updates as they become available.
Please direct any questions about this matter to NONINSTITUTIONAL_POLICY@medicaid.ohio.gov.
Medicare
Risk Adjustment Coding Webinar: Vascular Conditions
- August 9 @ 10am (EST)
- August 11 @ 9am (EST)
- August 15 @ 12noon (EST)
- August 15 @ 6pm (EST)
- August 17 @ 9am (EST)
Tips for Pediatric Risk Coding
- August 22 @ 10am (EST)
- August 24 @ 3pm (EST)
- August 30 @ 12noon (EST)
- August 30 @ 6pm (EST)
- September 1 @ 9am (EST)
MarketPlace
HHS Risk Adjustment – What do you need to know
- August 23 @ 10am (EST)
- August 25 @ 9am (EST)
- August 29 @ 12noon (EST)
- August 29 @ 6pm (EST)
- August 31 @ 3pm (EST)
Risk Adjustment 101 Medicare - Best Practices
- September 19 @ 10am (EST)
- September 21 @ 3pm (EST)
- September 26 @ 12noon (EST)
- September 28 @ 6pm (EST)
- September 29 @ 9am (EST)
Assertive Community Treatment (ACT) is an evidence-based model of delivering comprehensive community based behavioral health services to adults with certain serious and persistent mental illnesses who have not benefited from traditional outpatient treatment. ACT is a benefit available to Medicaid and MyCare Buckeye members who meet the criteria outlined in this revised clinical policy. The first 12 months of ACT services do not require prior authorization. This policy will be available on our Policy website page on September 24, 2023.
July 2023
For claims received on or after September 1, 2023, providers may experience a slight increase in written requests for medical records to determine if documentation supports services billed for ER surgical services where the follow up was not performed in the ER setting, and the correct modifier (54) was not included with the claim. These requests will come from Optum and will contain instructions for providing requested documentation. Should the requested documents not be returned, the claim(s) will be denied. Providers will have the ability to dispute findings through Optum directly in the event of a disagreement. Impacted lines of business are Medicare and Marketplace products.
Thank you for partnering with us to provide quality health care to our members, your patients.
Columbus Public Health is offering recommended childhood vaccines at no cost to ensure all children have a healthy and safe school year. City of Columbus and Worthington residents under the age of 18 who receive a school-required vaccine from Columbus Public Health at a specific vax cash clinic will receive a $100 visa gift card onsite when they are vaccinated, while supplies last. Various locations. You must make an appointment in advance.
Defects impacting delivery of 835 files
Ohio Department of Medicaid (ODM) is aware of issues affecting the provider community’s ability to consistently receive fee-for-service (FFS) 835 files since the Fiscal Intermediary (FI) launch on February 1. ODM understands the importance of timely and accurate data exchange and is committed to ensuring a smooth and reliable user experience. Over the last few months, ODM and its vendors have been identifying and correcting 835-related errors. As a result of the fixes, the majority of missing 835 details have been generated. A large batch was released on May 24, followed by a second batch released on June 26, and we expect another batch to be released today, July 7.
There is one remaining known issue that is currently being addressed. ODM has identified that this issue is primarily affecting hospitals claims. These files cannot be delivered since they failed SNIP edits at the Electronic Data Interchange (EDI). ODM vendors are working on implementing a fix in the coming 2-3 weeks.
If you have any questions or concerns about this issue, please contact Ohio Medicaid’s Integrated Helpdesk (IHD) at 800-686-1516 or email IHD@medicaid.ohio.gov. Representatives are available 8 a.m.-4:30 p.m. Eastern time Monday-Friday. We appreciate your understanding and your continued partnership.
Provider Network Management Disenrollment Quick Reference Guide is available
The Provider Network Management (PNM) Disenrollment Quick Reference Guide (QRG) offers step-by-step instructions on disenrolling a provider from Ohio Medicaid within the PNM module. These steps should only be completed if you wish to request the withdrawal of a provider’s enrollment with Ohio Medicaid. Once completed, the Medicaid ID assigned to that provider will no longer be active. The Disenrolling a Provider from Ohio Medicaid and other PNM QRGs are available on the PNM Learning tab.
Note: Disenrolling a provider is not the action you should take to remove or manage a provider’s affiliation with an organization or group.
For additional questions or technical support, please contact Ohio Medicaid’s Integrated Helpdesk (IHD) at 800-686-1516 or email IHD@medicaid.ohio.gov. Representatives are available 8 a.m.-4:30 p.m. Eastern time Monday-Friday.
To learn more about the PNM module and Centralized Credentialing, visit the PNM and Centralized Credentialing page on the Next Generation website.
June 2023
Requests to Providers for MyCare Contact Information
As part of Buckeye Health Plan’s normal business practice, we may reach out to our providers to gather the latest contact information on our MyCare members. Coordination of care between providers is part of our provider agreement, which allows us to work collaboratively to support our members.
Using submitted claims information, we touch base with various types of service providers to obtain or verify updated phone numbers, addresses and other information for our members. As the member’s health plan, we want to assure you that responding to us with this requested information is not a HIPAA violation. If you receive a request from us, we ask that you please provide this information at your earliest convenience. Thank you for all you do for our members, your patients.
If you have questions, please contact Provider Services at: 866.296.8731.
Update to CMS’ Three-Day Rule effective August 1, 2023
Centers for Medicare & Medicaid Services’ three-day rule, also known as the 72-hour rule guidelines require that hospitals bundle the technical component of all outpatient diagnostic and non-diagnostic services with the claim for an inpatient stay when services are furnished in the 3 days preceding an inpatient admission.
CMS requires that the 72 hours prior to an inpatient stay is bundled into the inpatient stay/claim and cannot be reimbursed separately.
- CMS requires that the 72 hours prior to an inpatient stay is bundled into the inpatient stay/claim and cannot be reimbursed separately
- Claims are matching based on provider TIN
- Professional claims are excluded
- Critical Access Hospital are excluded
- Any customization that was previously in place remains in place
Pilot Program Announcement
Buckeye Health is pleased to announce a pilot program in partnership with Ohio Sleep Treatment which specializes in treating Obstructive Sleep Apnea (OSA) with Oral Appliance Therapy (OAT). Buckeye is evaluating OAT as an "in lieu of" therapy for Sleep Apnea patients.
OAT is the main CPAP alternative and is recommended for mild and moderate OSA by the American Academy of Sleep Medicine (AASM). Ohio Sleep Treatment has offices in Westerville, Circleville, and Lancaster.
Providers who wish to learn more about Ohio Sleep Treatment may contact Rob Kibler directly at:
Email robk@sleeptreatmentoh.com
Direct - (614) 316-2062
Action required: PNM eLicense terminations
The Provider Network Management (PNM) module processed a monthly eLicense update on May 28 that matched the current license numbers entered in the PNM module as of that date. As a result, the system automatically terminated Ohio Medicaid providers with an expired license listed in the PNM module. On June 1, the PNM performed an automated script to reactivate affected providers in the system to allow time for providers to update this information before the next eLicense job.
What action do providers need to take?
Access the PNM module and confirm the license information is current before the next eLicense process runs on June 24. If the license information is not current, providers risk being terminated as an Ohio Medicaid provider. To prevent this from occurring in the future, all licensed Medicaid practitioners must keep their license date spans current in the PNM module.
For more information
For questions regarding this notice, please call the Ohio Department of Medicaid Integrated Helpdesk (IHD) at 800-686-1516 and select option 2; option 2 for provider enrollment. Representatives are available 8 a.m.-4:30 p.m. Eastern Time Monday-Friday.
Termination of Somatus Care Management Program for Patients with CKD/ESRD
Effective July 1, 2023, we will no longer partner with Somatus Inc., which provides care management services for members with chronic kidney disease (CKD) and end-stage renal disease (ESRD). Some of your patients may be affected by this change.
Consequently, some of your patients may be enrolled in our internal care management program, the Centene CKD Center of Excellence. This program will continue supporting care management needs for members with CKD and ESRD.
If you have any questions, please do not hesitate to contact your Provider Engagement Administrator.
Reminder: Non-agency (independent) waiver services providers must complete 12-hours of continuing education annually
Federal and state regulations require all non-agency waiver services providers to complete at least 12 hours of continuing education annually on or before your Ohio Department of Medicaid (ODM) contract anniversary date. If unknown, your ODM contract anniversary date can be found on the ‘Specialties’ page in the Provider Network Management (PNM) module. Your fulfillment of this rule requirement is reviewed when you participate in structural compliance reviews conducted by ODM’s Provider Oversight Contractor, Public Consulting Group (PCG).
What actions do I need to take?
- Complete 12 hours of continuing education each year prior to participating in the structural compliance review with PCG. PCG offers free continuing education courses on their website: PCG Training Materials. In addition, providers can take any qualified course or training that enhances the skills and competencies relevant to their job responsibilities and support person-centered service delivery.
- PCG will send an email one month prior to your ODM contract anniversary date, requesting a structural compliance review. Please reply promptly or call to schedule the date and time for the review.
For questions regarding this notice, please contact PCG via email at ohiohcbs@pcgus.com or via phone at 877-908-1746.
Ohio Department of Medicaid in-person site visits to resume July 1, 2023
The public health emergency has ended, and Ohio Department of Medicaid (ODM) is resuming site visits for initial provider enrollments and revalidations effective July 1, 2023. Site visits had been paused without impacting provider enrollment status. Site visits are part of ODM’s provider enrollment screening process and are required by state and federal regulations for certain provider types.
What action do I need to take?
Public Consulting Group (PCG), will be contacting you to schedule a site visit, which may be conducted either virtually or onsite. Please be responsive to PCG when they contact you.
For more information
For questions regarding this notice, please email OH_Provider_Screening@pcgus.com. For more information about provider enrollment and resources check out the Provider Enrollment page on the Medicaid website.
May 2023
Announcing: Buckeye Health Plan Receives Health Equity Accreditation from the National Committee for Quality Assurance
Buckeye Health Plan is delighted to share that we have received Health Equity Accreditation from the National Committee for Quality Assurance (NCQA). We are honored to be Ohio’s first managed care plan to receive Health Equity Accreditation from the NCQA. This accreditation recognizes Buckeye for providing culturally and linguistically sensitive services in more than 100 areas to eliminate healthcare disparities and support better health outcomes for our members.
During the accreditation process, Buckeye met and exceeded standards in key focus areas including community and member engagement, disparity reduction, provider training on cultural competency and health equity and diversity, equity, and inclusion. The NCQA accreditation process included a rigorous evaluation by a health plan expert.
Buckeye is thinking in new ways about the many systems that influence health, from education and housing to transportation and public safety. Health equity is central to our work across all lines of business, including our processes, practices, programs, and products. This accreditation gives us a solid framework to build upon to ensure we make meaningful differences in the lives of those we are honored to serve. Buckeye delivers high-quality care and service to help everyone live their healthiest life.
As you know, we continually review and update our payment and utilization policies to ensure that they are designed to comply with industry standards while delivering the best patient experience to our members. This is to inform you of the revision to existing Medicare and Marketplace effective 7-1-23.
Policy Updates
Policy Number | Policy Name | Policy Description | Line of Business |
CP.MP.100 | Allergy Testing and Therapy | Change codes 86160, 86161 and 86162 from not payable to NOT payable only when billed with the following diagnosis codes:, B44.81, H10.01* through H10.45, J30.1 through J30.9, J30.0, J31.0, J45.2* through J45.998 , L20.84 , L20.89, L20.9, L23.0 through L23.9*, L25.1 through L25.9, L27.0 through L27.9 , L50.0, L50.1, L50.6, L50.8, L50.9, L56.1, L56.2, L56.3, R06.2, T36.0X5A through T50.995S , T63.001* - T63.94*, T78.00X* through T78.1XXS, T78.49XA through T78.49XS , T80.52XA through T80.52XS, T88.6XXA through T88.6XXS , Z88.0 through Z88.9, Z91.010 through Z91.018, Add the following diagnosis codes as payable with 86003, 86005, 86008, 95004, 95017, 95018, 95024, 95027 and 95028. L20.0, L20.81-L20.83, L24.9, L30.2. Add CPT 86001 as NOT payable. | Medicare & Marketplace
|
CP.MP.97 | Testing for Select Genitourinary Conditions
| Added 0330U and 0352U as not med nec for members over age 13 (new code for July '22 with no utilization/cost data). Changed matching requirements for ICD-10 B37.3 to apply to B37.31 and B37.32 which together now replace B37.3. There will be no savings change from this edit. Changed CPT 87481 from not medically necessary in any circumstance to not med nec when paired with the following dx codes, and only applied to members 13 years and over. Required the same dx code matching for new code 0353U (with no utilization/cost data): B37.31, B37.32, L29.2, L29.3, N39.0,N72, N76.0, N76.1, N76.2, N76.3, N76.81, N76.89, N77.1, N89.8, N89.9, N90.89, N90.9, N91.0 –N91.5, N92.0, N93.0, N93.8, N93.9, N94.3, N94.4 – N94.6, N94.89, N94.9, O09.00-O09.03, O09.10-O09.13, O09.A0-O09.A3, O09.211-O09. 219,O09. 291-O09. 299,O09.30-O09.33,O09. 40-O09.43, O09.511-O09.519, O09.521- O09. 529, O09.611-O09.619, O09.621-O09.629, O09.70-O09.73, O09.811-O09.819, O09.821-O09.829, O09.891-O09.899, O09.90-O09.93, O23.511– O23.93, 00.00,Z00.8,Z01.419,Z11.3,Z11.51,Z22.330,Z23,Z30.011 – Z30.019,Z30.02, Z30.09,Z30.40 – Z30.9,Z32.00, Z33.1, Z34.00 – Z34.03, Z34.80 – Z34.83, Z34.90 – Z34.93, Z36.0-Z36.5, Z36.81-Z36.9, Z38.00 – Z38.01, Z38.30 – Z38.31, Z38.61 – Z38.69, Z39.0 – Z39.2, Z3A.00 – Z3A.49, Z72.51 – Z72.53, Z86.19, Z97.5
| Medicare & Marketplace
|
Effective July 1, 2023 Buckeye Health Plan will follow ODM guidance requiring use of the 33 modifier for the full reimbursement for filing the ePRAF. Buckeye will no longer reimburse providers the full payment unless the provider files the claim for the ePRAF with the 33 modifier as stipulated by ODM. Buckeye offered a grace period since ODM initially provided this guidance. Please refer to the chart below.
Payment for Completing the ePRAF
After completing the PRAF, submit a claim based on the guidelines below:
Reimbursement of ePRAF
Code + modifier | Description | Fee Schedule Amount* |
H1000 + 33 | Electronic PRAF Submission | $90.00 |
H1000 | Paper/Faxed version | $12.10 |
* Provider’s contracted rate will be applied to the fee schedule rate to determine final amount.
See more information on the PRAF and proper billing on our website.
Buckeye Health Plan's Payment Integrity department is implementing changes to the suite of unbundling edits with enhanced business rules to improve customer experience. Providers may see a reduction in unbundling edits starting in July of 2023, as Internal editing will reduce denials to only bundled modifier 59 code pair services which are clinically related.
We want to provide you with a heads up on upcoming enhancements to the Provider Portal Landing Page and a Pop-Up Survey you may encounter on the portal toward the end of June.
One of the most propelling reasons for the changes, is to make the page more accessible for our users. The portal is now 508 Compliant to come in line with the governments directive to ensure that disabled members of the public have comparable access. By doing this, we also ensure that everyone’s experience on the site is elevated. While none of the functionality will be changing, how the users interact with the information is changing. Below is an overview of the capabilities/modules you can expect:
- Notifications: Updated design to incorporate color coding, limit the characters allowed, and enabled the ability to set expiration dates.
- Personalized welcome with quick messages about the improved functionality.
- Admin Settings: Quick and easy access to core functionality of an Admin User.
- Quick Actions: Quick and easy access to Member Eligibility, New Claim, Recurring Claim, and Authorizations.
- Claims Overview: Dashboard of claims, segmented by Denied, Rejected, and Pending.
- Authorizations Overview: Quick access to inpatient and outpatient authorizations
- Useful links that are relevant to the user’s permissions and role.
In addition, you may encounter a Site Intercept Satisfaction Pop-Up Survey and/or a Feedback Tab Survey. To ensure you are experiencing the best possible use of the portal, we are placing short, automated pop-up intercepters to collect direct feedback from you about the portal experience.
More details and a Quick Start Guide will be coming soon.
Ohio Department of Medicaid will be hosting an opportunity to offer input and feedback on proposed updates to the CPC program administrative code rules (OAC 5160-19-01 and -02). ODM will be reviewing proposed changes, which will include updates to risk stratification, quality and efficiency metrics, and activities.
This meeting will take place on Thursday June 1, 2023 from 2:00 p.m. – 3:00 p.m. via GoToWebinar. If interested, please register here.
Medicare Member Plan Benefits Resume for Applicable COVID-19 Testing, Screening, and Treatment Services on May 12, 2023
Earlier this year, the Biden Administration announced that the federal Public Health Emergency (PHE) related to the COVID-19 pandemic will end on May 11, 2023.
During the PHE, we followed guidance from the Centers for Medicaid & Medicare Services (CMS) and instituted temporary waivers for select services. This action ensured that critical care could be quickly delivered to our members during a time of heightened need. Beginning May 12, 2023, these temporary waivers will expire, and our members’ Medicare plan benefits will be reinstated for the following services:
Sunsetting
Service | Member Liability | Prior Auth Needed? |
|---|---|---|
COVID-19 Testing and Screening (Administered by Provider) | Per member plan benefits | No |
COVID-19 Vaccinations | $0 member cost-share for vaccine administration* | No |
COVID-19 Monoclonal Antibody Treatments | $0 member cost-share for treatment administration* | Prior authorization only required for CPT code Q0221 |
*Vaccine ingredient cost is still covered directly by Medicare FFS.
Alongside these waivers, the Coronavirus Aid, Relief, and Economic Security (CARES) Act provided for a 20% increase to the inpatient prospective payment system (IPPS) Diagnosis Related Group (DRG) rate for COVID-19 patients for the duration of the public health emergency. This increase applied to claims that included the applicable COVID-19 ICD-10-CM diagnosis code and met the date of service requirement. When the PHE ends on May 11, 2023, these add-on payments will no longer be included for discharge dates of service as of May 12, 2023 and thereafter.
Wellcare by Allwell is committed to providing a smooth transition for both our members and providers as we resume business as usual. While we will continue to communicate any updates to our business practices directly to our provider partners, we always highly recommend that providers verify member eligibility, benefits, and prior authorization requirements before rendering services.
April 2023
End Date of Public Health Emergency (PHE) and PASRR Impact
In March 2020, the Ohio Department of Medicaid (ODM) made a number of operational changes to its Medicaid program in response to the COVID-19 public health emergency (PHE). These changes included taking advantage of the flexibilities offered to states including but not limited to allowing nursing facilities to delay the completion of the Preadmission Screening and Resident Review (PASRR) for 30-days.
On February 9, 2023, the Department of Health & Human Services announced that the PHE will end on May 11, 2023. While there were various flexibilities granted, the 30-day delay of PASRR Level I screenings and Level II evaluations will terminate on May 11, 2023.
As such, the Center for Medicare and Medicaid Services expect states to resume full PASRR activities in accordance with state PASRR rules (OAC 5160-3-15, OAC 5160-3-15.1 and OAC 5160-3-15.2) as of May 12, 2023. Therefore, providers must also return to the pre-PHE timeframes for completing PASRR requirements and related level of care requests. As a reminder, level of care determinations must not precede the date the PASRR requirements were met.
For additional questions, please submit them to PASRR@medicaid.ohio.gov
See OhioRISE
See Next Gen Contract Website Page
See EVV Website Page
Medicaid Providers Note:
We identified an issue where 835 files from Buckeye were not being received by OMES; therefore 835 files sent between February 1 to March 20, 2023, may be reprocessed which could cause duplication. Please make staff aware of this possibility to ensure the file is not posted a second time. If you have any questions, please reach out to Provider Services at 866-296-8731.
We apologize for any inconvenience this may cause and thank you in advance for your understanding.
March 2023
Claims Auditing – Custom Fitted or Custom Fabricated Prosthetics or Orthotics
On March 27, 2023, we notified our providers that we will begin performing additional prepayment claim reviews on July 1, 2023, using Optum’s Comprehensive Payment Integrity (CPI) tool. For Phase 1, claims received on or after July 1, 2023, providers may experience a slight increase in written requests for medical record submission prior to payment for Custom Fitted or Custom Fabricated Prosthetics or Orthotics. We will be requesting medical records to verify documentation that supports high-dollar custom DME codes billed by the provider. These requests will come from Optum and will contain instructions for providing the documentation.
Notification for Buckeye Health Plan, Wellcare By Allwell and Ambetter Providers:
We are committed to continuously improving our overall payment integrity solutions to prevent overpayments due to waste or abuse. This is a notification that we will begin performing additional prepayment claim reviews on July 1, 2023, using Optum’s Comprehensive Payment Integrity (CPI) tool. As a result of these prepayment claim reviews, providers may be asked for medical records and billing documents that support the charges billed.
We utilize widely acknowledged national guidelines for billing practices and support the concept of uniform billing for all payers. The prepayment claim reviews will look for overutilization of services or other practices that directly or indirectly result in unnecessary costs. A provider’s order must be present in the medical record to support all charges, along with clinical documentation to support the diagnosis and services or supplies billed.
If Optum’s review results in a finding, the provider will receive detailed instructions about how to submit requested documentation. Providers who do not submit the requested documentation may receive a technical denial, which will result in the claim being denied until the information required to adjudicate the claim is received.
If it is determined that a coding and/or payment adjustment is applicable, the provider will receive the appropriate claim adjudication. Providers retain their right to dispute results of reviews.
Please contact the applicable Provider Services listed on our home page or your Provider Engagement Administrator if you have any questions.
ODM Pause on Provider Agreement Revalidation/Recredentialing
The Ohio Department of Medicaid (ODM) paused provider agreement revalidations/recredentialing by pushing out all pending provider revalidation due dates in the Provider Network Management (PNM) module by 180 days. Dates were pushed out in the system during the week of February 6, 2023.
The updated due dates applied to all providers who had not begun the revalidation process prior to the week of February 6, including those that already displayed the “Begin Revalidation” button. This button indicated the provider had entered the 120-day period before revalidation is due. A provider’s revalidation date is indicated by the Medicaid Agreement End Date field in PNM. ODM issues a revalidation notice to the provider 120 days in advance of that date, including display of the “Begin Revalidation” button within their PNM account.
As a provider, what action do I need to take?
- If you do not see the “Begin Revalidation” button in the provider record, you do not need to do anything. The update will move the dates out another 180 days.
- If you do have the “Begin Revalidation” button, this means you are within the current 120-day window for revalidation.
EXCEPTION: If you started a revalidation prior to the week of February 6, 2023, it must be completed and submitted, as this change cannot be applied to providers that were already in the revalidation process.
Providers are strongly encouraged to avoid potential enrollment delays by submitting revalidation applications early in the process.
For more information: For technical support or assistance, contact Ohio Medicaid’s Integrated Helpdesk (IHD) at 800-686-1516 and follow the prompts for Provider Enrollment (option 2) or email IHD@medicaid.ohio.gov.
To learn more about the PNM module and Centralized Credentialing, visit the PNM and Centralized Credentialing page on the Next Generation website. ODM will provide additional guidance but the 5/11/23 date is the likely date that PHE is lifted which sets in motion various timelines to unwind from provider flexibilities. ODM will realign all revalidation dates accordingly and the MCE will access that information from the daily PMF.
See Next Gen Contract Website Page
See Behavioral Health Website page
Service Code | Service/Procedure Description | Comments |
A4239 (Formerly Code K0553, now retired) | Supplies, Continuous Glucose Monitoring | Allow 1 unit per month billed- PA required for over benefit limit only |
E2103 (Formerly Code K0554, now retired) | Receiver/Monitor, Continuous Glucose Monitor | Allow 1 monitor every 3 years- PA required for over benefit limit only |
A9277 | External Transmitter | Allow up to 2 per benefit year- PA required for over benefit limit only |
A9278 | External Receiver/Monitor | Allow 1 per benefit year- PA required for over benefit limit only |
See Next Gen Contract website page
February 2023
Effective April 1, 2023
Policy Number | Policy Name | Policy Description |
|---|---|---|
CP.MP.96 | Ambulatory EEG | Policy is being retired across all lines of business |
CP.MP.149 | Testing for Rupture of Fetal Membranes | Policy is being retired across all lines of business due to changes in standards of care. |
CP.MP.113 | Holter Monitors | Retiring for Medicare only as the LCDs are more lenient |
CP.MP.139 | Low-frequency ultrasound wound therapy | Retiring for Medicare only as the LCDs are more lenient |
CP.MP.152 | Measurement of Serum 1,25-dihydroxyvitamin D | Retiring for Medicare only as the LCDs are more lenient |
CP.MP.38 | Ultrasound in Pregnancy | Added new-for-2022 diagnosis codes as medical necessity/payable with 76811 |
CP.MP.134 | Evoked Potential Testing | Changed configuration so the edits don't apply to outpatient surgeries by matching revenue codes Retire for Medicare |
OC.UM.CP.0026 | Extended Ophthalmoscopy | Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92201/92202: , E09.37X1, E09.37X2, E09.37X3, E10.37X1, E10.37X2, E10.37X3, E11.37X1, E11.37X2, E11.37X3, H20.011, H20.012, H20.013, H20.021, H20.022, H20.023, H20.031, H20.032, H20.033, H20.041, H20.042, H20.043, H20.11, H20.12, H20.13, H20.21, H20.22, H20.23, H20.811, H20.812, H20.813, H20.01, H20.02, H20.03, H21.301, H21.302, H21.303, H21.311, H21.312, H21.313, H21.321, H21.322, H21.323, H21.341, H21.342, H21.343, H21.351, H21.352, H21.353, H21.531, H21.532, H21.533, H21.541, H21.542, H21.543, H21.551, H21.552, H21.553, H35.051, H35.052, H35.053, H35.21, H35.22, H35.23, H47.231, H47.232, H47.233, P07.01, P07.02, P07.03, P07.14, P07.15, P07.16, P07.17, P07.18, P07.21, P07.22, P07.23, P07.24, P07.25, P07.26, P07.31, P07.32, P07.33, P07.34, P07.35, P07.36, P07.37, P07.38, P07.39, Q85.01, Q85.02, Q85.03, S05.41XA, S05.41XD, S05.41XS, S05.42XA, S05.42XD, S05.42XS, T74.4XXA, T74.4XXD, T74.4XXS. Remove the following ICD-10 codes to the list of diagnoses that are payable when billed with 92201/92202:, H31.101, H31.102, H31.103, S05.71XA, S05.71XD, S05.71XS, S05.72XA, S05.72XD, S05.72XS Retire all edits for Medicare LOB as the LCDs are more lenient
|
OC.UM.CP.0028 | Fluorescein Angiography | Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92235: B20, B39.5, H33.111, H33.112, H33.113, H35.21, H35.22, H35.23, H35.361, H35.362, H35.363, H43.11, H43.12, H43.13, H43.821, H43.822, H43.823, Q14.8 Retire all edits for Medicare LOB as the LCDs are more lenient. |
OC.UM.CP.0029 | Fundus Photography | Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92250: Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92250: A51.43, A52.15, B25.8, G93.2, H33.121, H33.122, H33.123, Q14.8, S05.41XA, S05.41XD, S05.41XS, S05.42XA, S05.42XD, S05.42XS, S05.61XA, S05.61XD, S05.61XS, S05.62XA, S05.62XD, S05.62XS, Z85.840. Remove the following ICD-10 codes from the list of diagnoses that are payable when billed with 92250:, C69.01, C69.02, C69.11, C69.12, C69.51, C69.52, D49.89, Q87.1 Retire all edits for Medicare LOB as the LCDs are more lenient. |
OC.UM.CP.0043 | External Ocular Photography | Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92285: , A18.51, A18.54, A50.31, B00.53, B30.0, B30.1, B30.2, B30.3, C44.1922, C44.1991, C44.1992, C69.51, C69.52, C69.61, C69.62, C69.81, C69.82, D09.21, D09.22, D31.51, D31.52, H00.011, H00.012, H00.014, H00.015, H00.021, H00.022, H00.024, H00.025, H00.031, H00.032, H00.034, H00.035, H00.11, H00.12, H00.14, H00.15, H02.881, H02.882, H02.884, H02.885, H02.88A, H02.88B, H04.011, H04.012, H04.013, H04.021, H04.022, H04.023, H04.031, H04.032, H04.033, H04.111, H04.112, H04.113, H04.131, H04.132, H04.133, H04.161, H04.162, H04.163, H04.311, H04.312, H04.313, H04.321, H04.322, H04.323, H04.331, H04.332, H04.333, H04.411, H04.412, H04.413, H04.421, H04.422, H04.423, H04.431, H04.432, H04.433, H04.511, H04.512, H04.513, H04.521, H04.522, H04.523, H04.531, H04.532, H04.533, H05.011, H05.012, H05.013, H05.021, H05.022, H05.023, H05.031, H05.032, H05.033, H05.041, H05.042, H05.043, H05.111, H05.112, H05.113, H05.121, H05.122, H05.123, H05.211, H05.212, H05.213, H05.221, H05.222, H05.223, H05.231, H05.232, H05.233, H05.241, H05.242, H05.243, H05.251, H05.252, H05.253, H05.261, H05.262, H05.263, H05.311, H05.312, H05.313, H05.321, H05.322, H05.323, H05.331, H05.332, H05.333, H05.341, H05.342, H05.343, H05.351, H05.352, H05.353, H05.411, H05.412, H05.413, H05.421, H05.422, H05.423, H05.51, H05.52, H05.53, H05.811, H05.812, H05.813, H05.821, H05.822, H05.823, H16.241, H16.242, H16.243, H20.11, H20.12, H20.13, H20.21, H20.22, H20.23, H20.811, H20.812, H20.813, H20.821, H20.822, H20.823, H21.331, H21.332, H21.333, H21.561, H21.562, H21.563, H21.81, H27.111, H27.112, H27.113, H27.121, H27.122, H27.123, H27.131, H27.132, H27.133, H44.011, H44.012, H44.013, H44.111, H44.112, H44.113, H44.121, H44.122, H44.123, H44.131, H44.132, H44.133, S00.211A, S00.212A, S00.221A, S00.222A, S00.241A, S00.242A, S00.251A, S00.252A, S00.261A, S00.262A, S05.01XA, S05.01XD, S05.01XS, S05.02XA, S05.02XD, S05.02XS. Remove the following ICD-10 codes from the list of diagnoses that are payable when billed with 92285:, C44.131, H18.501, H18.502, H18.503 Retire all edits for Medicare LOB as the LCDs are more lenient. |
OC.UM.CP.0063 | Visual Field Testing | Add the following ICD-10 codes to the list of diagnoses that are payable when billed with 92081-3: , B58.01, C75.3, C79.31, D35.4, D43.3, E05.20, E05.21, E05.30, E05.31, E05.40, E05.41, G45.1, G45.2, G46.0, G46.1, G46.2, H02.211, H02.212, H02.214, H02.215, H02.21A, H02.21B, H02.21C, H02.221, H02.222, H02.224, H02.225, H02.22A, H02.22B, H02.22C, H02.231, H02.232, H02.234, H02.235, H02.23A, H02.23B, H02.23C, H02.841, H02.842, H02.844, H02.845, H02.851, H02.852, H02.854, H02.855, H05.121, H05.122, H05.123, H17.01, H17.02, H17.03, H17.11, H17.12, H17.13, H17.811, H17.812, H17.813, H17.821, H17.822, H17.823, H21.331, H21.332, H21.333, H31.011, H31.012, H31.013, H31.021, H31.022, H31.023, H33.121, H33.122, H33.123, H43.01, H43.02, H43.03, H43.11, H43.12, H43.13, H43.21, H43.22, H43.23, H43.311, H43.312, H43.313, H43.821, H43.822, H43.823, H44.21, H44.22, H44.23, H44.311, H44.312, H44.313, H44.411, H44.412, H44.413, H44.421, H44.422, H44.423, H44.431, H44.432, H44.433, H44.441, H44.442, H44.443, H44.511, H44.512, H44.513, H44.521, H44.522, H44.523, H44.531, H44.532, H44.533, H44.611, H44.612, H44.613, H44.621, H44.622, H44.623, H44.631, H44.632, H44.633, H44.641, H44.642, H44.643, H44.651, H44.652, H44.653, H44.691, H44.692, H44.693, H44.711, H44.712, H44.713, H44.721, H44.722, H44.723, H44.731, H44.732, H44.733, H44.741, H44.742, H44.743, H44.751, H44.752, H44.753, H44.791, H44.792, H44.793, H44.811, H44.812, H44.813, H44.821, H44.822, H44.823, H53.451, H53.452, H53.453, H57.02, H57.03, H57.04, H57.051, H57.052, H57.053, I60.2, I63.013, I63.033, I63.113, I63.133, I63.313, I63.323, I63.333, I63.343, I63.413, I63.423, I63.433, I63.443, I67.850, M31.6, S05.11XA, S05.11XD, S05.11XS, S05.12XA, S05.12XD, S05.12XS, S06.0X0A, S06.0X0D, S06.0X0S, S06.0X1A, S06.0X1D, S06.0X1S, S06.0X9A, S06.0X9D, S06.0X9S, S06.1X0A, S06.1X1A, S06.1X2A, S06.1X3A, S06.1X4A, S06.1X5A, S06.1X6A, S06.1X9A, S06.2X0A, S06.2X1A, S06.2X2A, S06.2X3A, S06.2X4A, S06.2X5A, S06.2X6A, S06.2X9A, S06.300A, S06.301A, S06.302A, S06.303A, S06.304A, S06.305A, S06.306A, S06.309A, S06.310A, S06.311A, S06.312A, S06.313A, S06.314A, S06.315A, S06.316A, S06.319A, S06.320A, S06.321A, S06.322A, S06.323A, S06.324A, S06.325A, S06.326A, S06.329A, S06.340A, S06.341A, S06.342A, S06.343A, S06.344A, S06.345A, S06.346A, S06.349A, S06.350A, S06.351A, S06.352A, S06.353A, S06.354A, S06.355A, S06.356A, S06.359A, S06.370A, S06.371A, S06.372A, S06.373A, S06.374A, S06.375A, S06.376A, S06.379A, S06.380A, S06.381A, S06.382A, S06.383A, S06.384A, S06.385A, S06.386A, S06.389A, S06.4X0A, S06.4X1A, S06.4X2A, S06.4X3A, S06.4X4A, S06.4X5A, S06.4X6A, S06.4X9A, S06.5X0A, S06.5X1A, S06.5X2A, S06.5X3A, S06.5X4A, S06.5X5A, S06.5X6A, S06.5X9A, S06.6X0A, S06.6X1A, S06.6X2A, S06.6X3A, S06.6X4A, S06.6X5A, S06.6X6A, S06.6X9A, S06.810A, S06.811A, S06.812A, S06.813A, S06.814A, S06.815A, S06.816A, S06.819A, S06.820A, S06.821A, S06.822A, S06.823A, S06.824A, S06.825A, S06.826A, S06.829A Retire all edits for Medicare LOB as the LCDs are more lenient. |
In response to your feedback, we have removed 16 services from our prior authorization list effective April 1, 2023:
PA Removals Eff April 1, 2023
Service Code | Service/Procedure Description | Comments |
|---|---|---|
81220 | Cystic Fibrosis Carrier Screen |
|
97110 | PT Services |
|
81420 | Fetal Chromosomal Screen |
|
81206 | Familial dysautonomia |
|
20550 | Injections ganglion cysts/plantar fascia |
|
20605 | Arthrocentesis, aspiration and/or injection, intermediate joint or bursa |
|
86832 | Antibody testing human leukocyte antigens (HLA) |
|
64885 | Nerve Graft Required PA for Non Par only. This will now be NO AUTH REQUIRED FOR ALL PROVIDERS |
|
97530 | Therapeutic Activities |
|
77002 | Fluoroscopic guidance for needle placement | No PA for All Providers |
81546 | Testing (genetic) with Thyroid Biopsies |
|
92507 | Speech Treatments | |
41899 | Facility charges around dental procedures done in hospital OR or Outpatient Surgery locations | No PA for All Providers |
00170 | Anesthesia charges for dental procedures done in hospital surgery, Outpatient Surgery locations |
|
Buckeye Health Plan is aligning with Ohio Department of Medicaid PA requirements for Continuous Glucose Monitoring supplies. PA requirements for network providers will be required if monthly/yearly amounts are more than the ODM recommended amounts below:
SERVICE CODE | SERVICE/PROCEDURE DESCRIPTION | COMMENTS |
|---|---|---|
K0553 | Supplies, Continuous Glucose Monitoring | Allow 1 unit per month billed- PA required for over benefit limit only |
A9277 | External Transmitter | Allow up to 2 per benefit year- PA required for over benefit limit only |
Buckeye Health Plan is adding Prior Authorization Requirements for the following code effective April 1, 2023:
PA Additions Effective April 1, 2023
SERVICE CODE | SERVICE/PROCEDURE DESCRIPTION | COMMENTS |
|---|---|---|
A6549 | Gradient Compression Stocking |
|
January 2023
See Next Gen Contract Website Page
See Next Gen Contract Website Page
See Next Gen Contract Website Page
See our EVV Website Page
December 2022
December 19, 2022: New version of the BH Provider Manual 1-1-2023
December 15, 2022: OhioRISE Mixed Services Protocol Updated December 8, 2022
December 15, 2022: Ambetter Providers - NCQA Letter
December 13, 2022: ODM Message - What to Expect on February 1, 2023
December 9, 2022: MITS Medicaid Payment Rates for CANS Assessments Will Increase January 1, 2023
December 9, 2022: December 9, 2022: “MITS BITS” is being replaced with “BH Bulletin”
December 9, 2022: MITS Claims Payment Error Resolved
December 7, 2022: ODM Message - Latest on Phase 3 move to February 1, 2023
- Anyone accessing the PNM or the SPBM secure web portal will need an OH|ID to log in and complete key administrative tasks and processes. The following resources are available to providers assist in setting up an account.
- Providers needing technical assistance should contact the Ohio Department of Medicaid Integrated Help Desk (IHD) at 1-800-686-1516. Hours of operation are Monday-Friday, 8 a.m. - 4:30 p.m. ET.
August 2022
(ODM apologizes for the error found in a communication sent out earlier this month. They stated that all demographic updates, including the CPC contact information, needed to be done in the MITS system by Aug 20 or the change will have to be held until Oct 1. The correct date is Aug 31st. Please see the corrected communication below.)
CPC Enrollment for the 2023 Program Year
The enrollment period for the CPC program is again slated for October. ODM anticipates sending out invitations to those who are eligible in early September 2022. Invitations will be sent via email to the CPC contact found in the MITS Secure Provider Portal.
Beginning Aug. 31, all provider demographic and agent maintenance update functionality will be closed for conversion of data in MITS. ENROLLED PROVIDERS SHOULD UPDATE THEIR DEMOGRAPHIC INFORMATION IN MITS BY AUGUST 31 or plan to hold updates until Oct. 1. It is therefore vital to make sure updates are completed by Aug. 31 to ensure all CPC invitations are received.
For assistance with how to update your demographic information, refer to the training video found on the ODM website. If additional assistance is needed, contact the Provider Hotline 800-686-1516.
July 2022
June 2022
- June 28, 2022: Coverage of psychiatric or substance use disorder inpatient admissions for youth
- June 21, 2022: Update to June 16, 2022 Practitioner Modifiers on Aetna OhioRISE Claims
- June 15, 21022 Informational Update - Extension of Reimbursement to non-VFC providers
- ODM has announced the availability of the recorded OhioRISE 1915(c) Home and Community Based Waiver training held on May 18. Click here for details and access.
May 2022
April 2022
- OhioRISE Home and Community Based Waiver training-Wednesday, May 18
- June 9 Comprehensive Primary Care (CPC) Webinar is on Big 5 initiatives
- Ohio Medicaid’s Next Generation program to launch July 1 with OhioRISE | Medicaid
- Effective April 13, 2022, Buckeye is following ODM guidance to rescind the lift of all prior authorizations and/or pre-certifications for all long-term acute care facility (hospital), skilled nursing facility (SNF), and Inpatient Rehabilitation facility (IRF-hospital) admissions
- New Short Stay Policy effective May 1
- ODM Freeze on MITS system process and update information

